Yonsei Med J.
2011 Jan;52(1):65-73. 10.3349/ymj.2011.52.1.65.
Granulocyte Colony Stimulating Factor Attenuates Hyperoxia-Induced Lung Injury by Down-Modulating Inflammatory Responses in Neonatal Rats
- Affiliations
-
- 1Department of Pediatrics, Pusan Paik Hospital, Inje University College of Medicine, Busan, Korea.
- 2Department of Pediatrics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. wonspark@skku.edu
- 3Samsung Biomedical Research Institute, Sungkyunkwan University School of Medicine, Seoul, Korea.
- KMID: 1106439
- DOI: http://doi.org/10.3349/ymj.2011.52.1.65
Abstract
- PURPOSE
Granulocyte colony stimulating factor (G-CSF) has been known to increase neutrophil production and have anti-inflammatory properties, but the effect of G-CSF on pulmonary system is in controversy. We investigated whether G-CSF treatment could attenuate hyperoxia-induced lung injury, and whether this protective effect is mediated by the down-modulation of inflammatory responses in a neonatal rat model.
MATERIALS AND METHODS
Newborn Sprague-Dawley rats (Orient Co., Seoul, Korea) were subjected to 14 days of hyperoxia (90% oxygen) beginning within 10 h after birth. G-CSF (20 microg/kg) was administered intraperitoneally on the fourth, fifth, and sixth postnatal days.
RESULTS
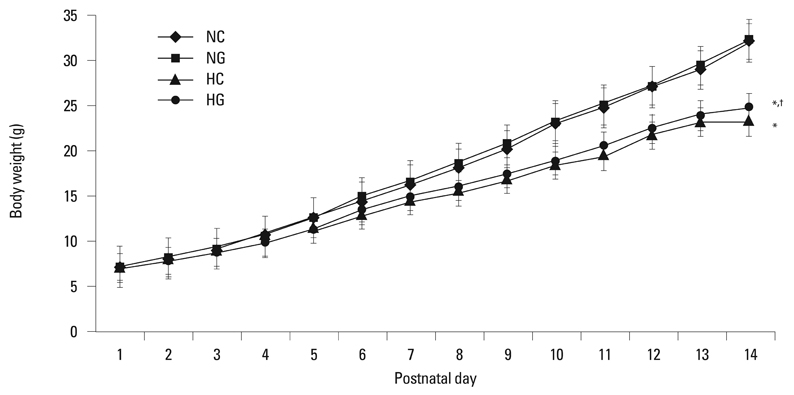
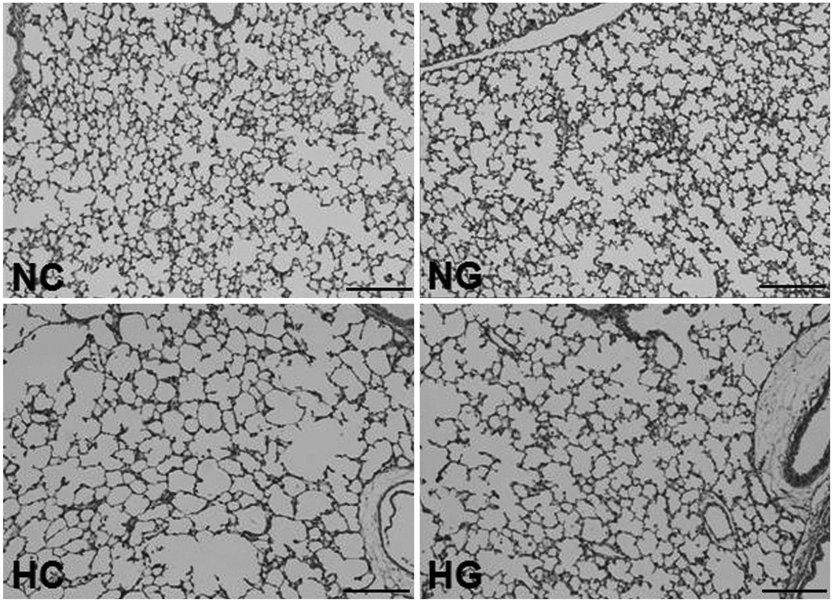
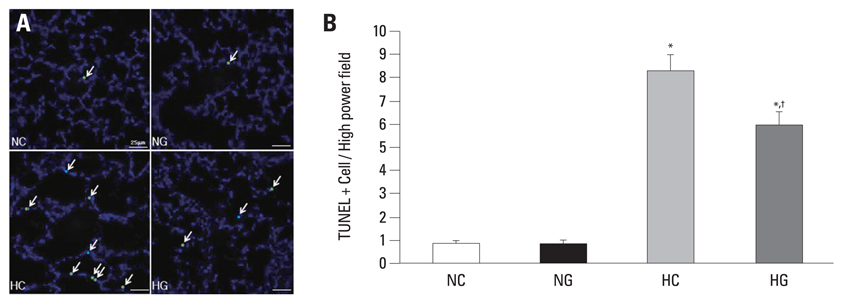
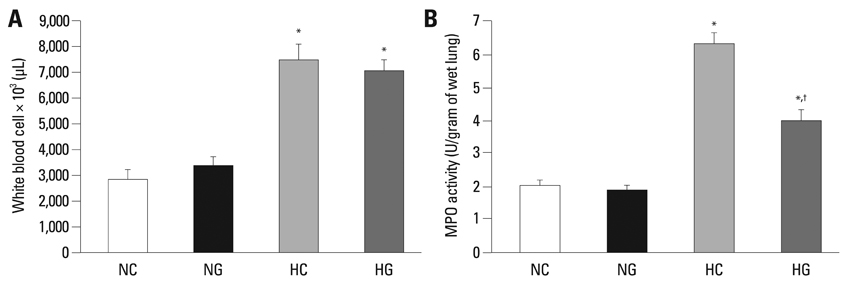
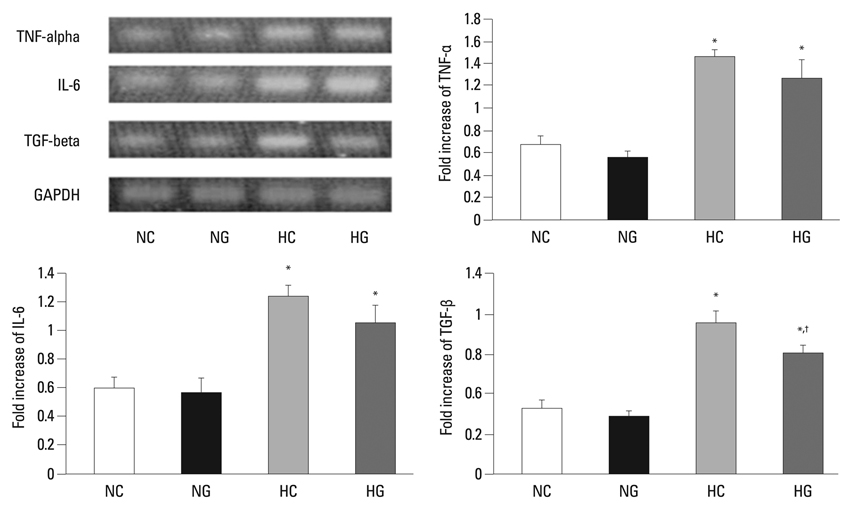
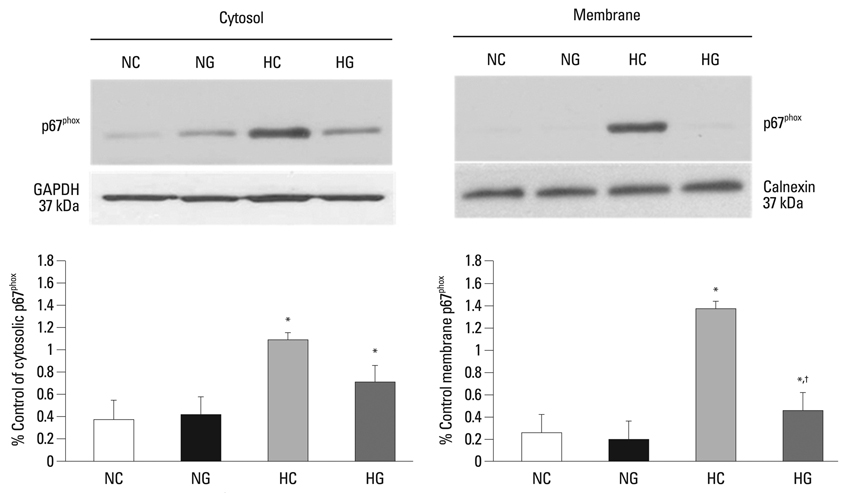
This treatment significantly improved hyperoxia-induced reduction in body weight gain and lung pathology such as increased mean linear intercept, mean alveolar volume, terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end labeling positive cells. Hyperoxia-induced activation of nicotinamide adenine dinucleotide phosphate oxidase, which is responsible for superoxide anion production, as evidenced by upregulation and membrane translocation of p67phox was significantly attenuated after G-CSF treatment, as were inflammatory responses such as increased myeloperoxidase activity and mRNA expression of transforming growth factor-beta. However, the attenuation of other proinflammatory cytokines such as tumor necrosis factor-alpha and interleukin-6 was not significant.
CONCLUSION
In sum, G-CSF treatment significantly attenuated hyperoxia-induced lung injury by down-modulating the inflammatory responses in neonatal rats.
Keyword
MeSH Terms
-
Animals
Animals, Newborn
Blotting, Western
Female
Granulocyte Colony-Stimulating Factor/*therapeutic use
Hyperoxia/*complications
In Situ Nick-End Labeling
Interleukin-6/genetics
Lung/*drug effects/*metabolism
Lung Injury/*drug therapy/etiology/genetics/metabolism
NADPH Oxidase/metabolism
Peroxidase/metabolism
Pregnancy
Random Allocation
Rats
Rats, Sprague-Dawley
Reverse Transcriptase Polymerase Chain Reaction
Transforming Growth Factor beta/genetics
Tumor Necrosis Factor-alpha/genetics
Weight Gain/drug effects
Figure
Reference
-
1. Kinsella JP, Greenough A, Abman SH. Bronchopulmonary dysplasia. Lancet. 2006. 367:1421–1431.
Article2. Short EJ, Klein NK, Lewis BA, Fulton S, Eisengart S, Kercsmar C, et al. Cognitive and academic consequences of bronchopulmonary dysplasia and very low birth weight: 8-year-old outcomes. Pediatrics. 2003. 112:e359.
Article3. Ehrenkranz RA, Walsh MC, Vohr BR, Jobe AH, Wright LL, Fanaroff AA, et al. Validation of the National Institutes of Health consensus definition of bronchopulmonary dysplasia. Pediatrics. 2005. 116:1353–1360.
Article4. Committee on Fetus and Newborn. Postnatal corticosteroids to treat or prevent chronic lung disease in preterm infants. Pediatrics. 2002. 109:330–338.5. Trial of late surfactant for prevention of bronchopulmonary dysplasia (TOLSIRF). from: URL: http://www.clinicaltrials.gov/ct2/show/nct01022580.6. Trial of late surfactant to prevent BPD: A pilot study in ventilated preterm neonates receiving inhaled nitric oxide (TOLSURF Pilot). from: URL: http://www.clinicaltrials.gov/ct2/show/nct00569530.7. Speer CP. Inflammation and bronchopulmonary dysplasia: a continuing story. Semin Fetal Neonatal Med. 2006. 11:354–362.
Article8. Juul S, Felderhoff-Mueser U. Epo and other hematopoietic factors. Semin Fetal Neonatal Med. 2007. 12:250–258.
Article9. Weiss SJ. Tissue destruction by neutrophils. N Engl J Med. 1989. 320:365–376.
Article10. Boneberg EM, Hartung T. Molecular aspects of anti-inflammatory action of G-CSF. Inflamm Res. 2002. 51:119–128.
Article11. Shyu WC, Lin SZ, Yang HI, Tzeng YS, Pang CY, Yen PS, et al. Functional recovery of stroke rats induced by granulocyte colony-stimulating factor-stimulated stem cells. Circulation. 2004. 110:1847–1854.
Article12. Carr R, Modi N, Doré C. G-CSF and GM-CSF for treating or preventing neonatal infections. Cochrane Database Syst Rev. 2003. CD003066.
Article13. Adach K, Suzuki M, Sugimoto T, Suzuki S, Niki R, Oyama A, et al. Granulocyte colony-stimulating factor exacerbates the acute lung injury and pulmonary fibrosis induced by intratracheal administration of bleomycin in rats. Exp Toxicol Pathol. 2002. 53:501–510.
Article14. Miura G, Awaya H, Matsumoto T, Tanaka N, Matsunaga N. Does granulocyte colony-stimulating factor exacerbate radiation-induced acute lung injury in rats? Radiat Med. 2000. 18:227–232.15. Wiedermann FJ, Mayr AJ, Hobisch-Hagen P, Fuchs D, Schobersberger W. Association of endogenous G-CSF with anti-inflammatory mediators in patients with acute respiratory distress syndrome. J Interferon Cytokine Res. 2003. 23:729–736.
Article16. Cooney TP, Thurlbeck WM. The radial alveolar count method of Emery and Mithal: a reappraisal 1--postnatal lung growth. Thorax. 1982. 37:572–579.
Article17. Snyder JM, Jenkins-Moore M, Jackson SK, Goss KL, Dai HH, Bangsund PJ, et al. Alveolarization in retinoic acid receptor-beta-deficient mice. Pediatr Res. 2005. 57:384–391.
Article18. Gray KD, Simovic MO, Chapman WC, Blackwell TS, Christman JW, May AK, et al. Endotoxin potentiates lung injury in cerulein-induced pancreatitis. Am J Surg. 2003. 186:526–530.
Article19. Babior BM. NADPH oxidase: an update. Blood. 1999. 93:1464–1476.
Article20. Robinson JM, Badwey JA. The NADPH oxidase complex of phagocytic leukocytes: a biochemical and cytochemical view. Histochem Cell Biol. 1995. 103:163–180.
Article21. Höhler B, Holzapfel B, Kummer W. NADPH oxidase subunits and superoxide production in porcine pulmonary artery endothelial cells. Histochem Cell Biol. 2000. 114:29–37.
Article22. Bland RD. Neonatal chronic lung disease in the post-surfactant era. Biol Neonate. 2005. 88:181–191.
Article23. Kunig AM, Balasubramaniam V, Markham NE, Morgan D, Montgomery G, Grover TR, et al. Recombinant human VEGF treatment enhances alveolarization after hyperoxic lung injury in neonatal rats. Am J Physiol Lung Cell Mol Physiol. 2005. 289:L529–L535.
Article24. McGrath-Morrow SA, Stahl J. Apoptosis in neonatal murine lung exposed to hyperoxia. Am J Respir Cell Mol Biol. 2001. 25:150–155.
Article25. Chanock SJ, el Benna J, Smith RM, Babior BM. The respiratory burst oxidase. J Biol Chem. 1994. 269:24519–24522.
Article26. Coalson JJ. Pathology of new bronchopulmonary dysplasia. Semin Neonatol. 2003. 8:73–81.
Article27. Görgen I, Hartung T, Leist M, Niehörster M, Tiegs G, Uhlig S, et al. Granulocyte colony-stimulating factor treatment protects rodents against lipopolysaccharide-induced toxicity via suppression of systemic tumor necrosis factor-alpha. J Immunol. 1992. 149:918–924.28. Gross-Weege W, Weiss M, Schneider M, Wenning M, Harms B, Dumon K, et al. Safety of a low-dosage Filgrastim (rhG-CSF) treatment in non-neutropenic surgical intensive care patients with an inflammatory process. Intensive Care Med. 1997. 23:16–22.
Article29. Knapp S, Hareng L, Rijneveld AW, Bresser P, van der Zee JS, Florquin S, et al. Activation of neutrophils and inhibition of the proinflammatory cytokine response by endogenous granulocyte colony-stimulating factor in murine pneumococcal pneumonia. J Infect Dis. 2004. 189:1506–1515.
Article30. Shyu WC, Lin SZ, Lee CC, Liu DD, Li H. Granulocyte colony-stimulating factor for acute ischemic stroke: a randomized controlled trial. CMAJ. 2006. 174:927–933.
Article31. Hartung T, Döcke WD, Gantner F, Krieger G, Sauer A, Stevens P, et al. Effect of granulocyte colony-stimulating factor treatment on ex vivo blood cytokine response in human volunteers. Blood. 1995. 85:2482–2489.
Article32. Donini M, Fontana S, Savoldi G, Vermi W, Tassone L, Gentili F, et al. G-CSF treatment of severe congenital neutropenia reverses neutropenia but does not correct the underlying functional deficiency of the neutrophil in defending against microorganisms. Blood. 2007. 109:4716–4723.
Article33. Carr R, Brocklehurst P, Doré CJ, Modi N. Granulocyte-macrophage colony stimulating factor administered as prophylaxis for reduction of sepsis in extremely preterm, small for gestational age neonates (the PROGRAMS trial): a single-blind, multicentre, randomised controlled trial. Lancet. 2009. 373:226–233.
Article34. Minnerup J, Sevimli S, Schäbitz WR. Granulocyte-colony stimulating factor for stroke treatment: mechanisms of action and efficacy in preclinical studies. Exp Transl Stroke Med. 2009. 1:2.
Article35. Kim BR, Shim JW, Sung DK, Kim SS, Jeon GW, Kim MJ, et al. Granulocyte stimulating factor attenuates hypoxic-ischemic brain injury by inhibiting apoptosis in neonatal rats. Yonsei Med J. 2008. 49:836–842.
Article36. Saugstad OD. Bronchopulmonary dysplasia-oxidative stress and antioxidants. Semin Neonatol. 2003. 8:39–49.
Article37. Bastian NR, Hibbs JB Jr. Assembly and regulation of NADPH oxidase and nitric oxide synthase. Curr Opin Immunol. 1994. 6:131–139.
Article38. Chang YS, Kim YJ, Yoo HS, Sung DK, Kim SY, Kang S, et al. Alpha-phenyl-N-tert-butylnitrone attenuates hyperoxia-induced lung injury by down-modulating inflammation in neonatal rats. Exp Lung Res. 2009. 35:234–249.
Article39. Delacourt C, d'Ortho MP, Macquin-Mavier I, Pezet S, Housset B, Lafuma C, et al. Oxidant-antioxidant balance in alveolar macrophages from newborn rats. Eur Respir J. 1996. 9:2517–2524.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Erythropoietin Attenuates Hyperoxia-Induced Lung Injury by Down-modulating Inflammation in Neonatal Rats
- Sweet Syndrome in a Child with Aplastic Anemia after Receiving Recombinant Granulocyte Colony-stimulating Factor
- Two cases of congenital agranulocytosis treated with recombinant human granulocyte colony-stimulating factor
- The effect of granulocyte colony-stimulating factor in chemotherapy of acute myelogenous leukemia
- Cell Division Cycle 2 Protects Neonatal Rats Against Hyperoxia-Induced Bronchopulmonary Dysplasia