J Vet Sci.
2006 Mar;7(1):73-77. 10.4142/jvs.2006.7.1.73.
Guided bone regeneration with beta-tricalcium phosphate and poly Llactide-co-glycolide-co-epsilon-caprolactone membrane in partial defects of canine humerus
- Affiliations
-
- 1Laboratory of Veterinary Surgery, College of Veterinary Medicine, Seoul National University, Seoul 151-742, Korea. ohkweon@snu.ac.kr
- 2Laboratory of Veterinary Pathology, College of Veterinary Medicine, Seoul National University, Seoul 151-742, Korea.
- 3Laboratory of Veterinary Radiology, College of Veterinary Medicine, Seoul National University, Seoul 151-742, Korea.
- 4Biomaterial Center, National Institute for Materials Science, Tsukuba, Ibaraki 305-0044, Japan.
- 5Department of Biomechanical Engineering, Institute of Biomaterials and Bioengineering, Tokyo Medical and Dental University, Chiyoda, Tokyo 101-0062, Japan.
- KMID: 1103558
- DOI: http://doi.org/10.4142/jvs.2006.7.1.73
Abstract
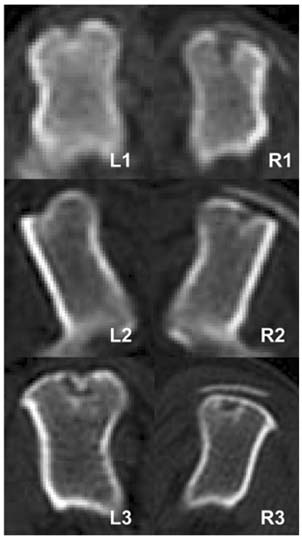
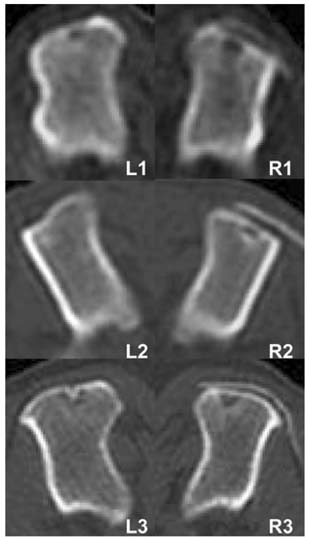
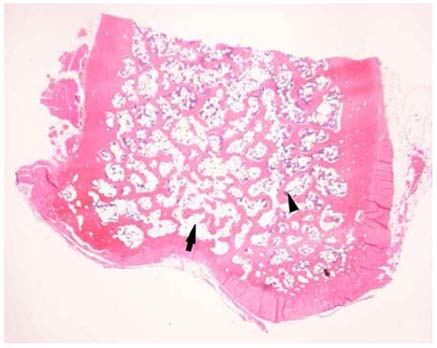
- This study was performed to evaluate the effect of betatricalcium phosphate and poly L-lactide-co-glycolide-coepsilon- caprolactone (TCP/PLGC) membrane in the repair of partial bone defects in canine proximal humerus. Three adult mixed-breed dogs were used during the experimental period. The length of the defect was quarter of the full length of humerus, and width of the defect was quarter of middle diameter of the lateral aspect of humerus. The humeri of each dog were divided into treatment (TCP/ PLGC) and control groups. The defect was covered with TCP/PLGC membrane in treatment group. To evaluate regeneration of the bone, computerized tomography (CT) and histopathologic examination were performed. The radiopaque lines were appeared at the original defect sites in TCP/PLGC group but below the original site in control at 4th week. Radiopacity and thickness of the defect sites, and radiopaque lines were more increased at 8th week than those of 4th week. Histopathologic findings revealed fibrous connective tissue migration into the defect and the migration inhibited the structure of new cortex to be placed in the original level in control whereas new cortex growth was found in the level of original line in TCP/ PLGC group. However, the new cortical bone in the TCP/ PLGC group was thinner and less organized than the adjacent intact cortex, and the amount of new cancellous bones were also scanty. The result suggested that TCP/ PLGC membrane is a good guided bone regeneration material to restore the original morphology of humerus in partial defect.
Keyword
MeSH Terms
Figure
Reference
-
1. Arbabi F. Structural Analysis and Behavior. 1991. NewYork: McGraw Hill;244–245.2. Artzi Z, Rohrer MD, Nemcovsky CE, Prasad HS, Tal H. Qualitative and quantitative expression of bovine bone mineral in experimental bone defects. Part 1: Description of a dog model and histological observations. J Periodontol. 2003. 74:1143–1152.
Article3. Blakemore ME. Fractures at cancellous bone graft donor sites. Injury. 1983. 14:519–522.
Article4. Clark CR, Morgan C, Sonstegard DA, Matthews LS. The effect of biopsy-hole shape and size on bone strength. J Bone Joint Surg. 1977. 59A:213–217.
Article5. Ferguson JF. Fracture of the humerus after cancellous bone graft harvesting in a dog. J Small Anim Pract. 1996. 37:232–234.6. Hockers T, Abensur D, Valentini P, Legrand R, Hammerle CHF. The combined use of bioresorbable membranes and xenografts around implants. A study in beagle dogs. Clin Oral Implants Res. 1999. 10:487–498.
Article7. Hulsse DA. Pathophysiology of autologous cancellous bone grafts. Compend Contin Educ Pract Vet. 1980. 11:136–142.8. Ignatius AA, Ohnmacht M, Claes LE, Kreidler J, Palm F. A composite polymer/tricalcium phosphate membrane for guided bone regeneration in maxillofacial surgery. J Biomed Mater Res. 2001. 58:564–569.
Article9. Jansen JA, de Ruijter JE, Jansen PTM, Paquay YGCJ. Histological evaluation of biodegradable polylactive/hydroxyapatite membrane. Biomaterials. 1995. 16:819–827.
Article10. Johnson KA. Cancellous bone graft collection from the tibia in dogs. Vet Surg. 1986. 15:334–338.
Article11. Johnson KA. Histologic features of the healing of bone graft donor sites in dogs. Am J Vet Res. 1988. 49:885–888.12. Koyama Y, Kikuchi M, Yamada T, Kanaya T, Hiroko N, Tsumoto M, Takakuda K, Miyairi H, Tanaka J. Guided bone regeneration with novel bioabsorbable membranes. JSME Int J (Series C). 2003. 46:1409–1416.
Article13. Kikuchi M, Suetsugu Y, Tanaka J, Akao M. Preparation and mechanical properties of calcium phosphate/copoly-L-lactide composites. J Mater Sci Mater Med. 1997. 8:361–364.14. Kikuchi M, Tanaka J, Koyama Y, Takakuda K. Cell culture test of TCP/CPLA composite. J Biomed Mater Res. 1999. 48:108–110.
Article15. Kikuchi M, Tanaka J. Chemical Interaction in beta-tricalcium phosphate/copolymerized poly-L-lactide composites. J Ceram Soc Japan. 2000. 108:642–645.
Article16. Kikuchi M, Koyama Y, Takakuda K, Miyairi H, Shirahama N, Tanaka J. In vitro change in mechanical strength of beta-tricalcium phosphate/copolymerized poly-L-lactide composites and their application for guided bone regeneration. J Biomed Mater Res. 2002. 62:265–272.
Article17. Kikuchi M, Koyama Y, Yamada T, Imamura Y, Okada T, Shirahama N, Akita K, Takakuda K, Tanaka J. Development of guided bone regeneration membrane composed of beta-tricalcium phosphate and poly (L-lactide-co-glycolide-co-epsilon-caprolactone) composites. Biomaterials. 2004. 25:5979–5986.
Article18. McGinnis M, Larsen P, Miloro M, Beck FM. Comparison of resorbable and nonresorbable guided bone regeneration materials: A preliminary study. Int J Oral Maxillofac Implants. 1998. 13:30–35.19. Millis LD, Martinez AS. Slatter DH, editor. Bone Grafts. Textbook of Small Animal Surgery. 2003. 3rd ed. Philadelphia: Saunders;1875–1891.20. Nyman S. Bone regeneration using the principle of guided tissue regeneration. J Clin Periodontol. 1991. 18:494–498.
Article21. Penwick RC, Mosier DA. Healing of canine autogenous cancellous bone graft donor sites. Vet Surg. 1991. 20:229–234.
Article22. Reilly DT, Burstein AH. Mechanical properties of cortical bone. J Bone Joint Surg. 1974. 56:1001–1022.23. Palmisano MP, Schrader SC. Premature closure of the proximal physis of the humerus in a dog as a result of harvesting a cancellous bone graft. J Am Vet Med Assoc. 1999. 215:1460–1462.24. Simpson AM. Fractures of the humerus. Clin Tech Small Anim Pract. 2004. 19:120–127.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of beta-Tricalcium Phosphate and Deproteinized Bovine Bone on Bone Formation in the Defects of Rat Calvaria
- The long-term study on the guided tissue regeneration with poly(alpha-hydroxy acid) membranes in beagle dogs
- Clinical Outcome of Beta-Tricalcium Phosphate Use for Bone Defects after Operative Treatment of Benign Tumors
- Effects of Tetracycline-loaded Poly(L-lactide) Barrier Membranes on Guided Bone Regeneration in Beagle Dog
- The Effect of Calcium-Phosphate Bovine Bone Powder on Guided Tissue Regeneration Using Biodegradable Membrane in Dogs