Clin Orthop Surg.
2019 Jun;11(2):233-236. 10.4055/cios.2019.11.2.233.
Clinical Outcome of Beta-Tricalcium Phosphate Use for Bone Defects after Operative Treatment of Benign Tumors
- Affiliations
-
- 1Department of Orthopedic Surgery, Kosin University Gospel Hospital, Kosin University College of Medicine, Busan, Korea. shchung@kosin.ac.kr
- KMID: 2445060
- DOI: http://doi.org/10.4055/cios.2019.11.2.233
Abstract
- BACKGROUND
We investigated the clinical outcome in patients whose cavitary bone defects were treated with beta-tricalcium phosphate (β-TCP) after surgical removal of benign tumors.
METHODS
Between March 2015 and December 2015, 20 patients who underwent operation for bone tumors were enrolled into this study and prospectively followed up for a median period of 28.1 months.
RESULTS
When the radiographic sign of complete resorption was defined as greater than 50% resorption of the allograft material accompanied by bone remodeling until 12 months, 55% of patients had complete resorption. Positive correlation between the filling volume and time needed for complete resorption was not found (p = 0.184).
CONCLUSIONS
Purified β-TCP could be a suitable choice as a bone graft substitute after the removal of benign bone tumors.
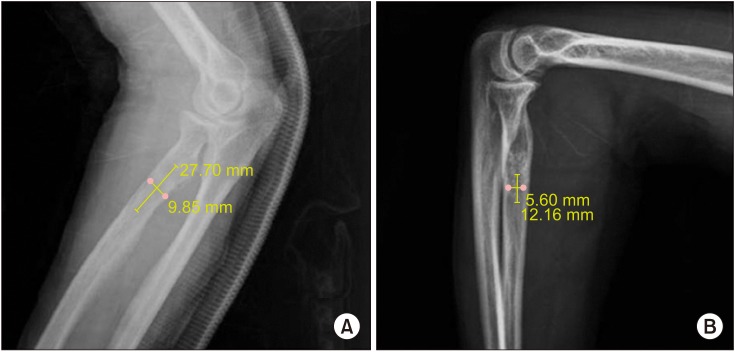
Figure
Reference
-
1. Arrington ED, Smith WJ, Chambers HG, Bucknell AL, Davino NA. Complications of iliac crest bone graft harvesting. Clin Orthop Relat Res. 1996; (329):300–309.
Article2. Banwart JC, Asher MA, Hassanein RS. Iliac crest bone graft harvest donor site morbidity: a statistical evaluation. Spine (Phila Pa 1976). 1995; 20(9):1055–1060. PMID: 7631235.3. Dong J, Uemura T, Shirasaki Y, Tateishi T. Promotion of bone formation using highly pure porous beta-TCP combined with bone marrow-derived osteoprogenitor cells. Biomaterials. 2002; 23(23):4493–4502. PMID: 12322969.4. Hollinger JO, Brekke J, Gruskin E, Lee D. Role of bone substitutes. Clin Orthop Relat Res. 1996; (324):55–65.
Article5. Laurencin C, Khan Y, El-Amin SF. Bone graft substitutes. Expert Rev Med Devices. 2006; 3(1):49–57. PMID: 16359252.
Article6. Bucholz RW. Nonallograft osteoconductive bone graft substitutes. Clin Orthop Relat Res. 2002; (395):44–52. PMID: 11937865.
Article7. Galois L, Mainard D, Delagoutte JP. Beta-tricalcium phosphate ceramic as a bone substitute in orthopaedic surgery. Int Orthop. 2002; 26(2):109–115. PMID: 12078872.8. Nicholas RW, Lange TA. Granular tricalcium phosphate grafting of cavitary lesions in human bone. Clin Orthop Relat Res. 1994; (306):197–203.9. Vaccaro AR. The role of the osteoconductive scaffold in synthetic bone graft. Orthopedics. 2002; 25(5 Suppl):s571–s578. PMID: 12038844.
Article10. Botez P, Sirbu P, Simion L, Munteanu F, Antoniac I. Application of a biphasic macroporous synthetic bone substitutes CERAFORM®: clinical and histological results. Eur J Orthop Surg Traumatol. 2009; 19(6):387–395.
Article11. Hirata M, Murata H, Takeshita H, Sakabe T, Tsuji Y, Kubo T. Use of purified beta-tricalcium phosphate for filling defects after curettage of benign bone tumours. Int Orthop. 2006; 30(6):510–513. PMID: 16736145.
Article12. Mano JF, Sousa RA, Boesel LF, Neves NM, Reis RL. Bioinert, biodegradable and injectable polymeric matrix composites for hard tissue replacement: state of the art and recent developments. Compos Sci Technol. 2004; 64(6):789–817.
Article13. Naito K, Obayashi O, Mogami A, Itoi A, Kaneko K. Fracture of the calcium phosphate bone cement which used to enchondroma of the hand: a case report. Eur J Orthop Surg Traumatol. 2008; 18(5):405–408.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of beta-Tricalcium Phosphate and Deproteinized Bovine Bone on Bone Formation in the Defects of Rat Calvaria
- Union Rates of Local Autobone and beta-Tricalcium Phosphate Mixed Graft in Lumbar Posterolateral Fusion
- Interference of Union after the Use of Beta-Tricalcium Phosphate Block in High Tibial Osteotomy
- Alterations of Gene Expression by Beta-tricalcium Phosphate in Osteoblast-like MG63 Cells
- The BMPs expression and histomorphometric study of beta-TCP /rhBMP-2 Grafting on the rabbit cranial bone defects