Korean J Radiol.
2009 Dec;10(6):559-567. 10.3348/kjr.2009.10.6.559.
Estimation of Pulmonary Motion in Healthy Subjects and Patients with Intrathoracic Tumors Using 3D-Dynamic MRI: Initial Results
- Affiliations
-
- 1Department of Radiology, German Cancer Research Center Heidelberg, Germany. christian.plathow@uniklinikfreiburg. de
- 2Department of Medical and Biological Informatics, German Cancer Research Center Heidelberg, Germany.
- 3Department of Nuclear Medicine, University of Freiburg, Germany.
- 4Department of Internal Medicine, Clinic of Thoracic Disease, Germany.
- 5Department of Diagnostic Radiology, Clinic of Thoracic Disease, Germany.
- 6Department of Diagnostic and Interventional Radiology, University of Heidelberg, Germany.
- KMID: 1102558
- DOI: http://doi.org/10.3348/kjr.2009.10.6.559
Abstract
OBJECTIVE
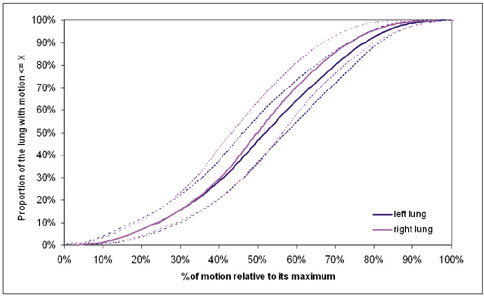
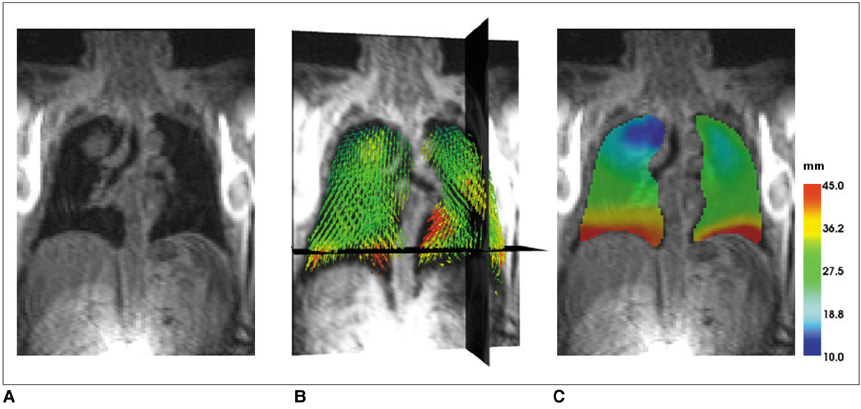
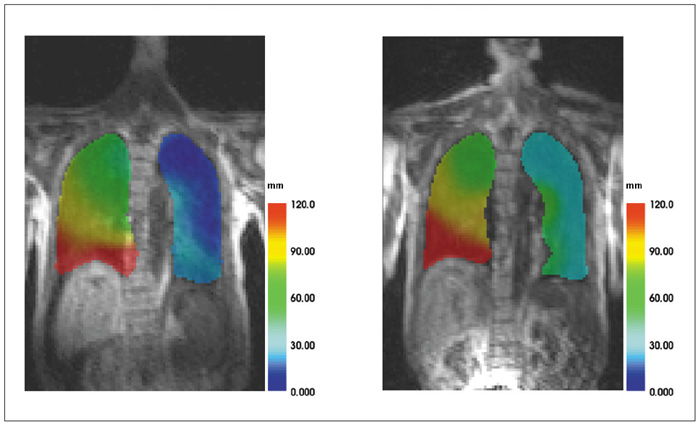
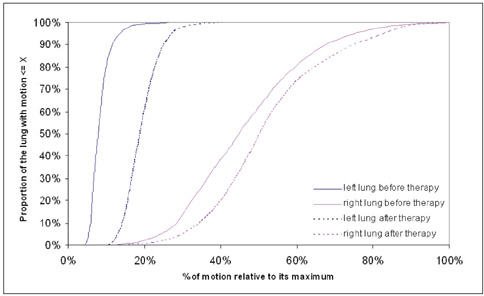
To estimate a new technique for quantifying regional lung motion using 3D-MRI in healthy volunteers and to apply the technique in patients with intra- or extrapulmonary tumors. MATERIALS AND METHODS: Intraparenchymal lung motion during a whole breathing cycle was quantified in 30 healthy volunteers using 3D-dynamic MRI (FLASH [fast low angle shot] 3D, TRICKS [time-resolved interpolated contrast kinetics]). Qualitative and quantitative vector color maps and cumulative histograms were performed using an introduced semiautomatic algorithm. An analysis of lung motion was performed and correlated with an established 2D-MRI technique for verification. As a proof of concept, the technique was applied in five patients with non-small cell lung cancer (NSCLC) and 5 patients with malignant pleural mesothelioma (MPM). RESULTS: The correlation between intraparenchymal lung motion of the basal lung parts and the 2D-MRI technique was significant (r = 0.89, p < 0.05). Also, the vector color maps quantitatively illustrated regional lung motion in all healthy volunteers. No differences were observed between both hemithoraces, which was verified by cumulative histograms. The patients with NSCLC showed a local lack of lung motion in the area of the tumor. In the patients with MPM, there was global diminished motion of the tumor bearing hemithorax, which improved siginificantly after chemotherapy (CHT) (assessed by the 2D- and 3D-techniques) (p < 0.01). Using global spirometry, an improvement could also be shown (vital capacity 2.9 +/- 0.5 versus 3.4 L +/- 0.6, FEV1 0.9 +/- 0.2 versus 1.4 +/- 0.2 L) after CHT, but this improvement was not significant. CONCLUSION: A 3D-dynamic MRI is able to quantify intraparenchymal lung motion. Local and global parenchymal pathologies can be precisely located and might be a new tool used to quantify even slight changes in lung motion (e.g. in therapy monitoring, follow-up studies or even benign lung diseases).
MeSH Terms
Figure
Reference
-
1. Petty TL, Silvers GW, Stanford RE. Mild emphysema is associated with reduced elastic recoil and increased lung size but not with air-flow limitation. Am Rev Respir Dis. 1987. 136:867–871.2. Clausen JL. The diagnosis of emphysema, chronic bronchitis and asthma. Clin Chest Med. 1990. 11:405–416.3. Suga K, Tsukuda T, Awaya H, Takano K, Koike S, Matsunaga N, et al. Impaired respiratory mechanics in pulmonary emphysema: evaluation with dynamic breathing MRI. J Magn Reson Imaging. 1999. 10:510–520.4. Plathow C, Ley S, Fink C, Puderbach M, Heilmann M, Zuna I, et al. Evaluation of chest motion and volumetry during the breathing cycle by dynamic MRI in healthy subjects: comparison with pulmonary function tests. Invest Radiol. 2004. 39:202–209.5. Plathow C, Fink C, Ley S, Puderbach M, Eichinger M, Schmähl A, et al. Measurement of diaphragmatic length during the breathing cycle by dynamic MRI: comparison between healthy adults and patients with an intrathoracic tumor. Eur Radiol. 2004. 14:1392–1399.6. Axel L, Dougherty L. MR imaging of motion with spatial modulation of magnetization. Radiology. 1989. 171:841–845.7. Napadow VJ, Mai V, Bankier A, Gilbert RJ, Edelman R, Chen Q. Determination of regional pulmonary parenchymal strain during normal respiration using spin inversion tagged magnetization MRI. J Magn Reson Imaging. 2001. 13:467–474.8. Fink C, Ley S, Kroeker R, Requardt M, Kauczor HU, Bock M. Time-resolved contrast-enhanced three-dimensional magnetic resonance angiography of the chest: combination of parallel imaging with view sharing (TREAT). Invest Radiol. 2005. 40:40–48.9. Plathow C, Schoebinger M, Fink C, Hof H, Debus J, Meinzer HP, et al. Quantification of lung tumor volume and rotation at 3D dynamic parallel MR imaging with view sharing: preliminary results. Radiology. 2006. 240:537–545.10. Sarrut D, Boldea V, Ayadi M, Badel JN, Ginestet C, Clippe S, et al. Nonrigid registration method to assess reproducibility of breath-holding with ABC in lung cancer. Int J Radiat Oncol Biol Phys. 2005. 61:594–607.11. Gee J, Sundaram T, Hasegawa I, Uematsu H, Hatabu H. Characterization of regional pulmonary mechanics from serial magnetic resonance imaging data. Acad Radiol. 2003. 10:1147–1152.12. Plathow C, Schoebinger M, Fink C, Ley S, Puderbach M, Eichinger M, et al. Evaluation of lung volumetry using dynamic three-dimensional magnetic resonance imaging. Invest Radiol. 2005. 40:173–179.13. Plathow C, Ley S, Fink C, Puderbach M, Hosch W, Schmähl A, et al. Analysis of intrathoracic tumor mobility during the whole breathing cycle by dynamic MRI. Int J Radiat Oncol Biol Phys. 2004. 59:952–959.14. Vogelzang NJ, Rusthoven JJ, Symanowski J, Denham C, Kaukel E, Ruffie P, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol. 2003. 21:2636–2644.15. Du J, Bydder M. High-resolution time-resolved contrast-enhanced MR abdominal and pulmonary angiography using a spiral-TRICKS sequence. Magn Reson Med. 2007. 58:631–635.16. Cootes TG, Taylor CJ, Cooper DH, Graham J. Active shape models - their training and application. Comput Vis Image Underst. 1995. 61:38–59.17. Heimann T, Münzing S, Meinzer HP, Wolf I. A shape-guided deformable model with evolutionary algorithm initialization for 3D soft tissue segmentation. Inf Process Med Imaging. 2007. 20:1–12.18. Heimann T, Wolf I, Meinzer HP. Automatic generation of 3D statistical shape models with optimal landmark distributions. Methods Inf Med. 2007. 46:275–281.19. Wolf I, Vetter M, Wegner I, Böttger T, Nolden M, Schöbinger M, et al. The medical imaging interaction toolkit. Med Image Anal. 2005. 9:594–604.20. Plathow C, Klopp M, Schoebinger M, Thieke C, Fink C, Puderbach M, et al. Monitoring of lung motion in patients with malignant pleural mesothelioma using two-dimensional and three-dimensional dynamic magnetic resonance imaging: comparison with spirometry. Invest Radiol. 2006. 41:443–448.21. Hof H, Herfarth KK, Münter M, Hoess A, Motsch J, Wannenmacher M, et al. Stereotactic single-dose radiotherapy of stage I non-small-cell lung cancer (NSCLC). Int J Radiat Oncol Biol Phys. 2003. 56:335–341.22. Hof H, Herfarth KK, Münter M, Essig M, Wannenmacher M, Debus J. The use of the multislice CT for the determination of respiratory lung tumor movement in stereotactic single-dose irradiation. Strahlenther Onkol. 2003. 179:542–547.23. Shirato H, Oita M, Fujita K, Watanabe Y, Miyasaka K. Feasibility of synchronization of real-time tumor-tracking radiotherapy and intensity-modulated radiotherapy from viewpoint of excessive dose from fluoroscopy. Int J Radiat Oncol Biol Phys. 2004. 60:335–341.24. Plathow C, Fink C, Ley S, Puderbach M, Eichinger M, Zuna I, et al. Measurement of tumor diameter-dependent mobility of lung tumors by dynamic MRI. Radiother Oncol. 2004. 73:349–354.25. Remouchamps VM, Letts N, Yan D, Vicini FA, Moreau M, Zielinski JA, et al. Three-dimensional evaluation of intra- and interfraction immobilization of lung and chest wall using active breathing control: a reproducibility study with breast cancer patients. Int J Radiat Oncol Biol Phys. 2003. 57:968–978.26. Eibel R, Tuengerthal S, Schoenberg SO. The role of new imaging techniques in diagnosis and staging of malignant pleural mesothelioma. Curr Opin Oncol. 2003. 15:131–138.27. Plathow C, Hof H, Kuhn S, Puderbach M, Ley S, Biederer J, et al. Therapy monitoring using dynamic MRI: analysis of lung motion and intrathoracic tumor mobility before and after radiotherapy. Eur Radiol. 2006. 16:1942–1950.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Incentive Spirometry on Respiratory Motion in Healthy Subjects Using Cine Breathing Magnetic Resonance Imaging
- Comparison of interference from eccentric movements of dental crowns fabricated via dynamic jaw motion tracking and conventional methods: a double-blind clinical study
- Enhancing mediastinal tumors: CT evaluation
- Development of External Linkage Type of Spine Motion Analyer and Thoracic & Lumbar Motion Analysis in Normal Subjects
- Advanced Methods in Dynamic Contrast Enhanced Arterial Phase Imaging of the Liver