Investig Magn Reson Imaging.
2019 Mar;23(1):1-16. 10.13104/imri.2019.23.1.1.
Advanced Methods in Dynamic Contrast Enhanced Arterial Phase Imaging of the Liver
- Affiliations
-
- 1Clinical Research Institute, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. yoonckim@skku.edu
- KMID: 2442228
- DOI: http://doi.org/10.13104/imri.2019.23.1.1
Abstract
- Dynamic contrast enhanced (DCE) magnetic resonance (MR) imaging plays an important role in non-invasive detection and characterization of primary and metastatic lesions in the liver. Recently, efforts have been made to improve spatial and temporal resolution of DCE liver MRI for arterial phase imaging. Review of recent publications related to arterial phase imaging of the liver indicates that there exist primarily two approaches: breath-hold and free-breathing. For breath-hold imaging, acquiring multiple arterial phase images in a breath-hold is the preferred approach over conventional single-phase imaging. For free-breathing imaging, a combination of three-dimensional (3D) stack-of-stars golden-angle sampling and compressed sensing parallel imaging reconstruction is one of emerging techniques. Self-gating can be used to decrease respiratory motion artifact. This article introduces recent MRI technologies relevant to hepatic arterial phase imaging, including differential subsampling with Cartesian ordering (DISCO), golden-angle radial sparse parallel (GRASP), and X-D GRASP. This article also describes techniques related to dynamic 3D image reconstruction of the liver from golden-angle stack-of-stars data.
Keyword
MeSH Terms
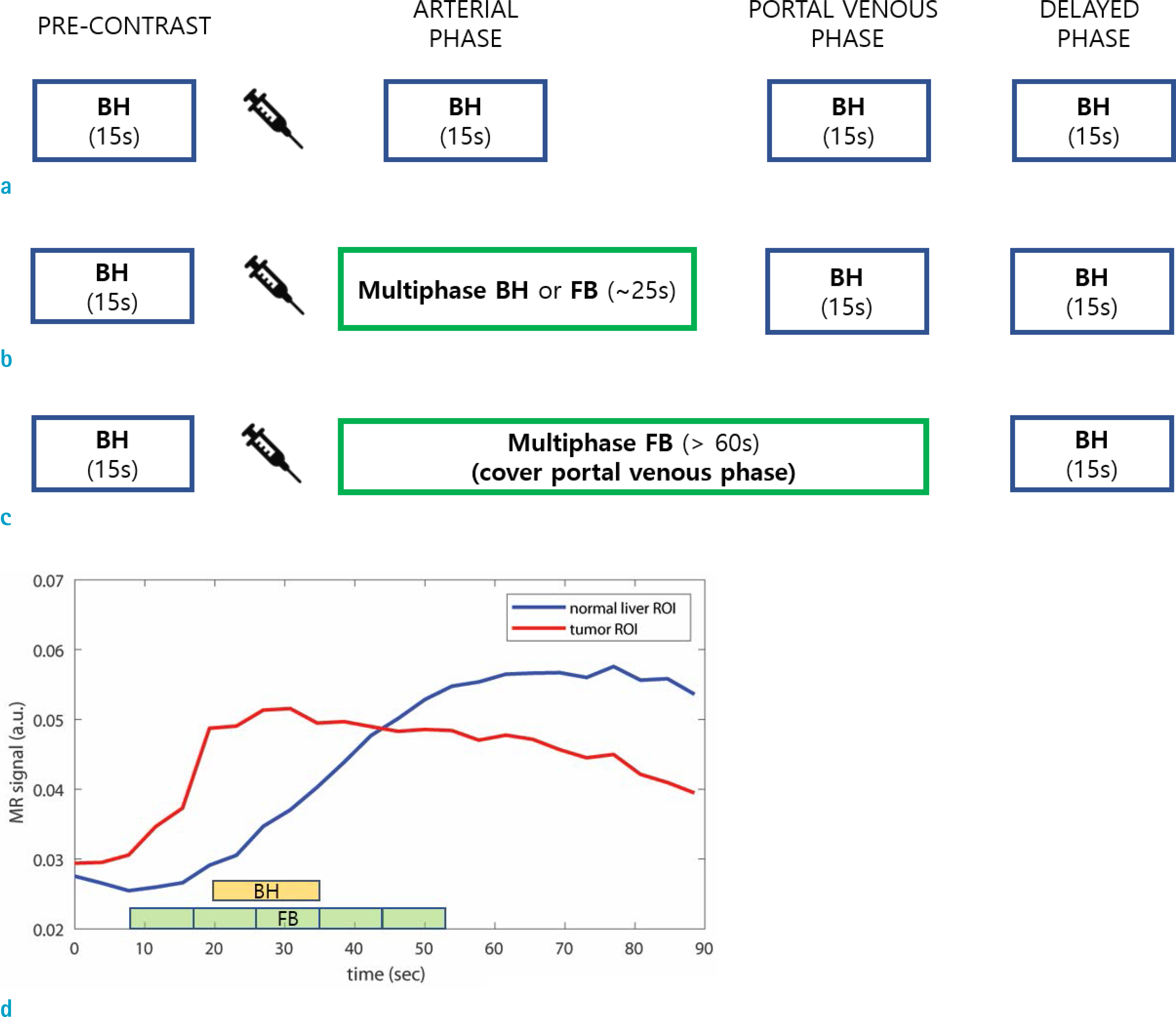
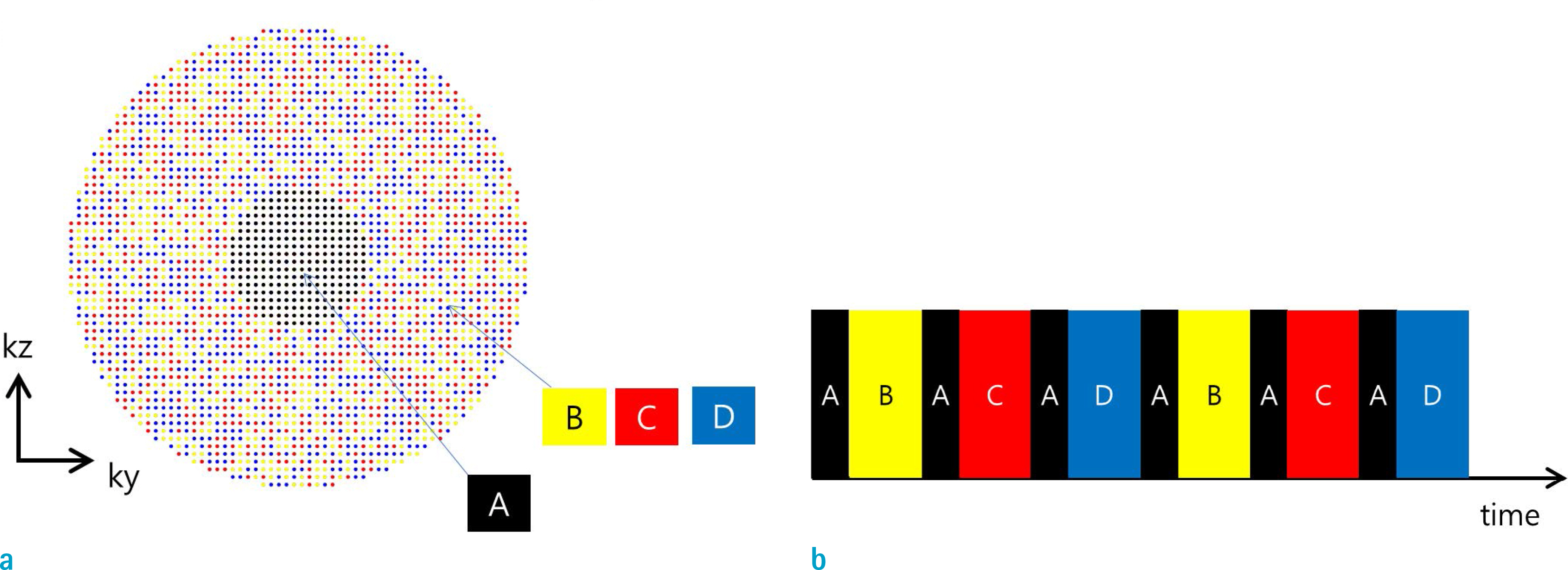
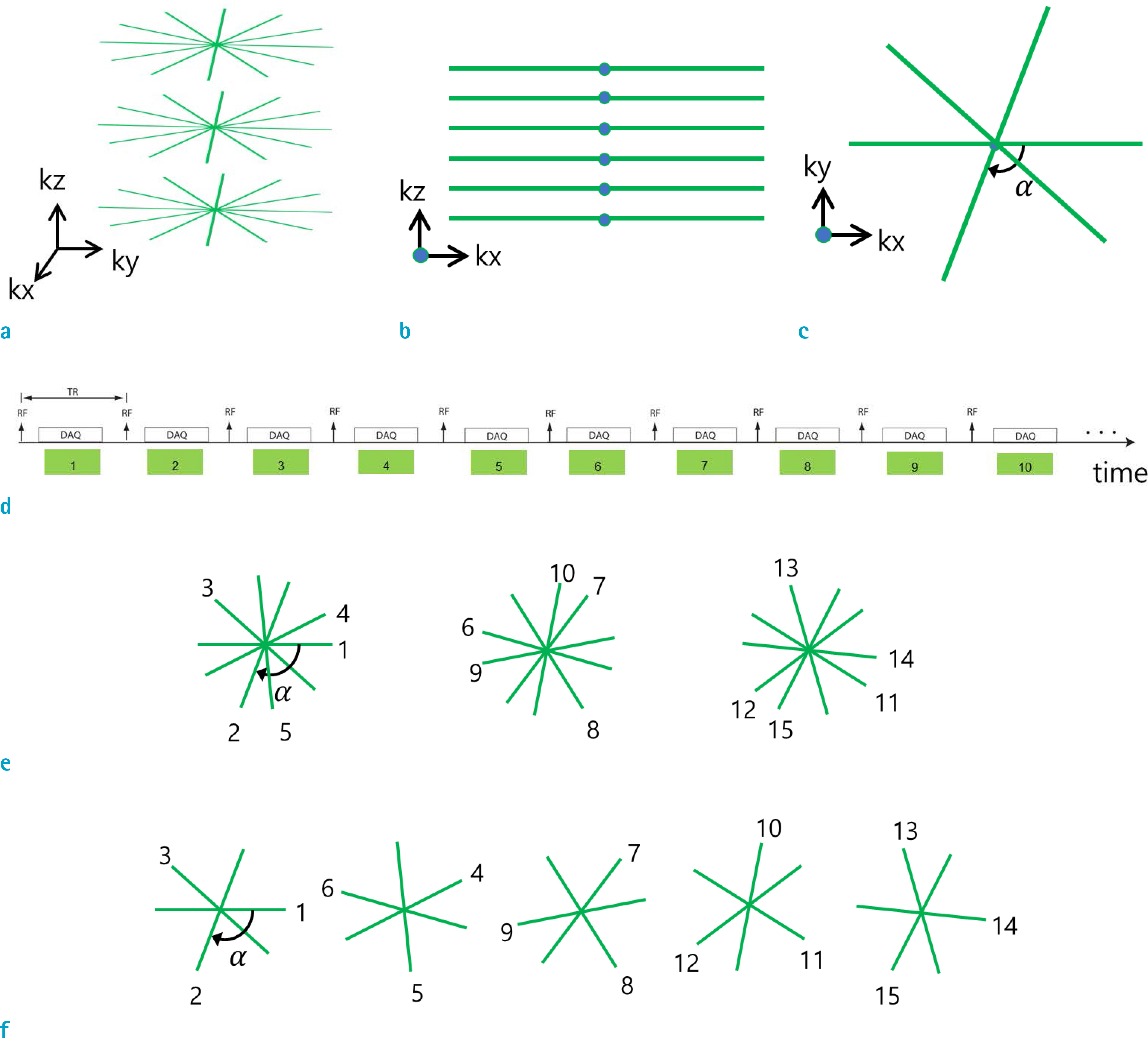
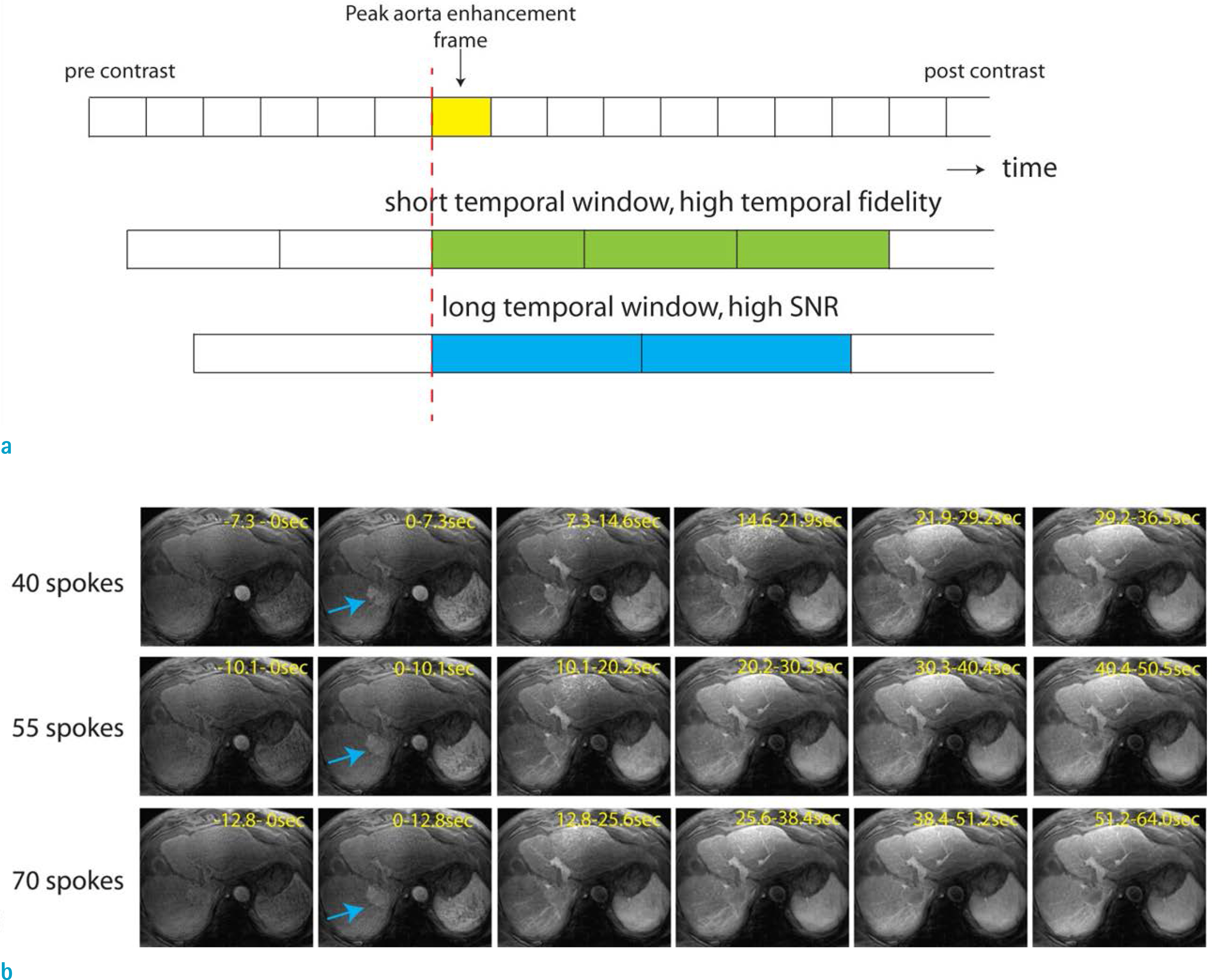
Figure
Cited by 1 articles
-
Rapid Imaging: Recent Advances in Abdominal MRI for Reducing Acquisition Time and Its Clinical Applications
Jeong Hee Yoon, Marcel Dominik Nickel, Johannes M. Peeters, Jeong Min Lee
Korean J Radiol. 2019;20(12):1597-1615. doi: 10.3348/kjr.2018.0931.
Reference
-
References
1. Gomaa AI, Khan SA, Toledano MB, Waked I, Taylor-Robinson SD. Hepatocellular carcinoma: epidemiology, risk factors and pathogenesis. World J Gastroenterol. 2008; 14:4300–4308.
Article2. Mittal S, El-Serag HB. Epidemiology of hepatocellular carcinoma: consider the population. J Clin Gastroenterol. 2013; 47(Suppl):S2–6.3. Ringe KI, Husarik DB, Sirlin CB, Merkle EM. Gadoxetate disodium-enhanced MRI of the liver: part 1, protocol optimization and lesion appearance in the noncirrhotic liver. AJR Am J Roentgenol. 2010; 195:13–28.
Article4. Cruite I, Schroeder M, Merkle EM, Sirlin CB. Gadoxetate disodium-enhanced MRI of the liver: part 2, protocol optimization and lesion appearance in the cirrhotic liver. AJR Am J Roentgenol. 2010; 195:29–41.
Article5. Bruix J, Sherman M. American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011; 53:1020–1022.
Article6. Nakamura S, Nakaura T, Kidoh M, et al. Timing of the hepatic arterial phase at Gd-EOB-DTPA-enhanced hepatic dynamic MRI: comparison of the test-injection and the fixed-time delay method. J Magn Reson Imaging. 2013; 38:548–554.
Article7. Pietryga JA, Burke LM, Marin D, Jaffe TA, Bashir MR. Respiratory motion artifact affecting hepatic arterial phase imaging with gadoxetate disodium: examination recovery with a multiple arterial phase acquisition. Radiology. 2014; 271:426–434.
Article8. Saranathan M, Rettmann DW, Hargreaves BA, Clarke SE, Vasanawala SS. DIfferential Subsampling with Cartesian Ordering (DISCO): a high spatiotemporal resolution Dixon imaging sequence for multiphasic contrast enhanced abdominal imaging. J Magn Reson Imaging. 2012; 35:1484–1492.
Article9. Hope TA, Saranathan M, Petkovska I, Hargreaves BA, Herfkens RJ, Vasanawala SS. Improvement of gadoxetate arterial phase capture with a high spatiotemporal resolution multiphase three-dimensional SPGR-Dixon sequence. J Magn Reson Imaging. 2013; 38:938–945.
Article10. Ichikawa S, Motosugi U, Oishi N, et al. Ring-like enhancement of hepatocellular carcinoma in gadoxetic acid-enhanced multiphasic hepatic arterial phase imaging with differential subsampling with cartesian ordering. Invest Radiol. 2018; 53:191–199.
Article11. Clarke SE, Saranathan M, Rettmann DW, Hargreaves BA, Vasanawala SS. High resolution multi-arterial phase MRI improves lesion contrast in chronic liver disease. Clin Invest Med. 2015; 38:E90–99.
Article12. Ikram NS, Yee J, Weinstein S, et al. Multiple arterial phase MRI of arterial hypervascular hepatic lesions: improved arterial phase capture and lesion enhancement. Abdom Radiol (NY). 2017; 42:870–876.
Article13. Fujinaga Y, Ohya A, Tokoro H, et al. Radial volumetric imaging breathhold examination (VIBE) with k-space weighted image contrast (KWIC) for dynamic gadoxetic acid (Gd-EOB-DTPA)-enhanced MRI of the liver: advantages over Cartesian VIBE in the arterial phase. Eur Radiol. 2014; 24:1290–1299.
Article14. Chandarana H, Feng L, Block TK, et al. Free-breathing contrast-enhanced multiphase MRI of the liver using a combination of compressed sensing, parallel imaging, and golden-angle radial sampling. Invest Radiol. 2013; 48:10–16.
Article15. Feng L, Grimm R, Block KT, et al. Golden-angle radial sparse parallel MRI: combination of compressed sensing, parallel imaging, and golden-angle radial sampling for fast and flexible dynamic volumetric MRI. Magn Reson Med. 2014; 72:707–717.
Article16. Feng L, Axel L, Chandarana H, Block KT, Sodickson DK, Otazo R. XD-GRASP: Golden-angle radial MRI with reconstruction of extra motion-state dimensions using compressed sensing. Magn Reson Med. 2016; 75:775–788.
Article17. Chandarana H, Feng L, Ream J, et al. Respiratory motion-resolved compressed sensing reconstruction of free-breathing radial acquisition for dynamic liver magnetic resonance imaging. Invest Radiol. 2015; 50:749–756.
Article18. Choi JY, Lee JM, Sirlin CB. CT and MR imaging diagnosis and staging of hepatocellular carcinoma: part I. Development, growth, and spread: key pathologic and imaging aspects. Radiology. 2014; 272:635–654.
Article19. Choi JY, Lee JM, Sirlin CB. CT and MR imaging diagnosis and staging of hepatocellular carcinoma: part II. Extracellular agents, hepatobiliary agents, and ancillary imaging features. Radiology. 2014; 273:30–50.
Article20. Motosugi U, Bannas P, Sano K, Reeder SB. Hepatobiliary MR contrast agents in hypovascular hepatocellular carcinoma. J Magn Reson Imaging. 2015; 41:251–265.
Article21. Yoon JH, Lee JM, Yu MH, Kim EJ, Han JK. Triple arterial phase MR imaging with gadoxetic acid using a combination of contrast enhanced time robust angiography, keyhole, and viewsharing techniques and two-dimensional parallel imaging in comparison with conventional single arterial phase. Korean J Radiol. 2016; 17:522–532.
Article22. Davenport MS, Bashir MR, Pietryga JA, Weber JT, Khalatbari S, Hussain HK. Dose-toxicity relationship of gadoxetate disodium and transient severe respiratory motion artifact. AJR Am J Roentgenol. 2014; 203:796–802.
Article23. Huh J, Kim SY, Yeh BM, et al. Troubleshooting arterial-phase MR images of gadoxetate disodium-enhanced liver. Korean J Radiol. 2015; 16:1207–1215.
Article24. Davenport MS, Viglianti BL, Al-Hawary MM, et al. Comparison of acute transient dyspnea after intravenous administration of gadoxetate disodium and gadobenate dimeglumine: effect on arterial phase image quality. Radiology. 2013; 266:452–461.
Article25. Davenport MS, Caoili EM, Kaza RK, Hussain HK. Matched within-patient cohort study of transient arterial phase respiratory motion-related artifact in MR imaging of the liver: gadoxetate disodium versus gadobenate dimeglumine. Radiology. 2014; 272:123–131.
Article26. Yoon JH, Lee JM, Yu MH, et al. Evaluation of transient motion during gadoxetic acid-enhanced multiphasic liver magnetic resonance imaging using free-breathing golden-angle radial sparse parallel magnetic resonance imaging. Invest Radiol. 2018; 53:52–61.
Article27. Min JH, Kim YK, Kang TW, et al. Artifacts during the arterial phase of gadoxetate disodium-enhanced MRI: multiple arterial phases using viewsharing from two different vendors versus single arterial phase imaging. Eur Radiol. 2018; 28:3335–3346.
Article28. Hope TA, Petkovska I, Saranathan M, Hargreaves BA, Vasanawala SS. Combined parenchymal and vascular imaging: high spatiotemporal resolution arterial evaluation of hepatocellular carcinoma. J Magn Reson Imaging. 2016; 43:859–865.
Article29. Lustig M, Donoho D, Pauly JM. Sparse MRI: the application of compressed sensing for rapid MR imaging. Magn Reson Med. 2007; 58:1182–1195.
Article30. Kaltenbach B, Bucher AM, Wichmann JL, et al. Dynamic liver magnetic resonance imaging in free-breathing: feasibility of a cartesian T1-weighted acquisition technique with compressed sensing and additional self-navigation signal for hard-gated and motion-resolved reconstruction. Invest Radiol. 2017; 52:708–714.31. Weiss J, Notohamiprodjo M, Martirosian P, et al. Self-gated 4D-MRI of the liver: initial clinical results of continuous multiphase imaging of hepatic enhancement. J Magn Reson Imaging. 2018; 47:459–467.
Article32. Peters DC, Derbyshire JA, McVeigh ER. Centering the projection reconstruction trajectory: reducing gradient delay errors. Magn Reson Med. 2003; 50:1–6.
Article33. Block KT. Advanced methods for radial data sampling in MRI. Ph.D. thesis, Georg-August-Universitaet Goettingen,. 2008.34. Block KT, Uecker M, Frahm J. Undersampled radial MRI with multiple coils. Iterative image reconstruction using a total variation constraint. Magn Reson Med. 2007; 57:1086–1098.
Article35. Song HK, Dougherty L. Dynamic MRI with projection reconstruction and KWIC processing for simultaneous high spatial and temporal resolution. Magn Reson Med. 2004; 52:815–824.
Article36. Winkelmann S, Schaeffter T, Koehler T, Eggers H, Doessel O. An optimal radial profile order based on the golden ratio for time-resolved MRI. IEEE Trans Med Imaging. 2007; 26:68–76.
Article37. Chan RW, Ramsay EA, Cheung EY, Plewes DB. The influence of radial undersampling schemes on compressed sensing reconstruction in breast MRI. Magn Reson Med. 2012; 67:363–377.
Article38. Hedderich DM, Weiss K, Spiro JE, et al. Clinical evaluation of free-breathing contrast-enhanced T1w MRI of the Liver using pseudo golden angle radial k-space sampling. Rofo. 2018; 190:601–609.
Article39. Kajita K, Goshima S, Noda Y, et al. Thin-slice free-breathing pseudo-golden-angle radial stack-of-stars with gating and tracking T1-weighted acquisition: an efficient gadoxetic acid-enhanced hepatobiliary-phase imaging alternative for patients with unstable breath holding. Magn Reson Med Sci. 2019; 18:4–11.40. Brodsky EK, Bultman EM, Johnson KM, et al. High-spatial and high-temporal resolution dynamic contrast-enhanced perfusion imaging of the liver with time-resolved three-dimensional radial MRI. Magn Reson Med. 2014; 71:934–941.
Article41. Block KT, Frahm J. Spiral imaging: a critical appraisal. J Magn Reson Imaging. 2005; 21:657–668.
Article42. Agrawal MD, Spincemaille P, Mennitt KW, et al. Improved hepatic arterial phase MRI with 3-second temporal resolution. J Magn Reson Imaging. 2013; 37:1129–1136.
Article43. Xu B, Spincemaille P, Chen G, et al. Fast 3D contrast enhanced MRI of the liver using temporal resolution acceleration with constrained evolution reconstruction. Magn Reson Med. 2013; 69:370–381.
Article44. Breuer FA, Blaimer M, Heidemann RM, Mueller MF, Griswold MA, Jakob PM. Controlled aliasing in parallel imaging results in higher acceleration (CAIPIRINHA) for multislice imaging. Magn Reson Med. 2005; 53:684–691.
Article45. Breuer FA, Blaimer M, Mueller MF, et al. Controlled aliasing in volumetric parallel imaging (2D CAIPIRINHA). Magn Reson Med. 2006; 55:549–556.
Article46. Kim BS, Lee KR, Goh MJ. New imaging strategies using a motion-resistant liver sequence in uncooperative patients. Biomed Res Int. 2014; 2014:142658.
Article47. Wright KL, Harrell MW, Jesberger JA, et al. Clinical evaluation of CAIPIRINHA: comparison against a GRAPPA standard. J Magn Reson Imaging. 2014; 39:189–194.
Article48. Michaely HJ, Morelli JN, Budjan J, et al. CAIPIRINHA-Dixon-TWIST (CDT)-volume-interpolated breathhold examination (VIBE): a new technique for fast time-resolved dynamic 3-dimensional imaging of the abdomen with high spatial resolution. Invest Radiol. 2013; 48:590–597.49. Yu MH, Lee JM, Yoon JH, Kiefer B, Han JK, Choi BI. Clinical application of controlled aliasing in parallel imaging results in a higher acceleration (CAIPIRINHA)volumetric interpolated breathhold (VIBE) sequence for gadoxetic acid-enhanced liver MR imaging. J Magn Reson Imaging. 2013; 38:1020–1026.
Article50. Park YS, Lee CH, Kim IS, et al. Usefulness of controlled aliasing in parallel imaging results in higher acceleration in gadoxetic acid-enhanced liver magnetic resonance imaging to clarify the hepatic arterial phase. Invest Radiol. 2014; 49:183–188.
Article51. Beck GM, De Becker J, Jones AC, von Falkenhausen M, Willinek WA, Gieseke J. Contrast-enhanced timing robust acquisition order with a preparation of the longitudinal signal component (CENTRA plus) for 3D contrast-enhanced abdominal imaging. J Magn Reson Imaging. 2008; 27:1461–1467.
Article52. Eggers H, Bornert P. Chemical shift encoding-based water-fat separation methods. J Magn Reson Imaging. 2014; 40:251–268.
Article53. Eggers H, Brendel B, Duijndam A, Herigault G. Dual-echo Dixon imaging with flexible choice of echo times. Magn Reson Med. 2011; 65:96–107.
Article54. Berglund J, Ahlstrom H, Johansson L, Kullberg J. Two-point dixon method with flexible echo times. Magn Reson Med. 2011; 65:994–1004.
Article55. Benkert T, Feng L, Sodickson DK, Chandarana H, Block KT. Free-breathing volumetric fat/water separation by combining radial sampling, compressed sensing, and parallel imaging. Magn Reson Med. 2017; 78:565–576.
Article56. Pandharipande PV, Krinsky GA, Rusinek H, Lee VS. Perfusion imaging of the liver: current challenges and future goals. Radiology. 2005; 234:661–673.
Article57. Thng CH, Koh TS, Collins DJ, Koh DM. Perfusion magnetic resonance imaging of the liver. World J Gastroenterol. 2010; 16:1598–1609.
Article58. Sourbron S, Sommer WH, Reiser MF, Zech CJ. Combined quantification of liver perfusion and function with dynamic gadoxetic acid-enhanced MR imaging. Radiology. 2012; 263:874–883.
Article59. Chen Y, Lee GR, Wright KL, et al. Free-breathing liver perfusion imaging using 3-dimensional through-time spiral generalized autocalibrating partially parallel acquisition acceleration. Invest Radiol. 2015; 50:367–375.
Article60. Chandarana H, Block TK, Ream J, et al. Estimating liver perfusion from free-breathing continuously acquired dynamic gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid-enhanced acquisition with compressed sensing reconstruction. Invest Radiol. 2015; 50:88–94.
Article61. Hammernik K, Klatzer T, Kobler E, et al. Learning a variational network for reconstruction of accelerated MRI data. Magn Reson Med. 2018; 79:3055–3071.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Test-bolus injection for optimization of arterial phase imaging during contrast-enhanced hepatic MR imaging
- Current Limitations and Potential Breakthroughs for the Early Diagnosis of Hepatocellular Carcinoma
- Detectability of Hepatocellular Carcinoma: Comparison of Gd-DT PA-Enhanced and SPIO-Enhanced MR Imaging
- Hepatic Cavernous Hemangioma in Cirrhotic Liver: Imaging Findings
- Troubleshooting Arterial-Phase MR Images of Gadoxetate Disodium-Enhanced Liver