Korean J Lab Med.
2010 Apr;30(2):126-132. 10.3343/kjlm.2010.30.2.126.
Performance Characteristics of the UniCel DxI 800 Immunoassay for the Maternal Serum Quadruple Test, Including Median Values for Each Week of Gestation, in Korean Women
- Affiliations
-
- 1Department of Laboratory Medicine, Yonsei University College of Medicine, Seoul, Korea. jeongho@yuhs.ac
- 2Division of Immunology, Department of Laboratory Medicine, Seoul Medical Science Institute, Seoul Clinical Laboratories, Seoul, Korea.
- KMID: 1096797
- DOI: http://doi.org/10.3343/kjlm.2010.30.2.126
Abstract
- BACKGROUND
Maternal serum prenatal quadruple screening includes testing for alpha-fetoprotein (AFP), human chorionic gonadotrophin (hCG), unconjugated estriol (uE3), and dimeric inhibin A (DIA). We evaluated quadruple screening using an automated platform and looked for any ethnic differences in the median values of each marker.
METHODS
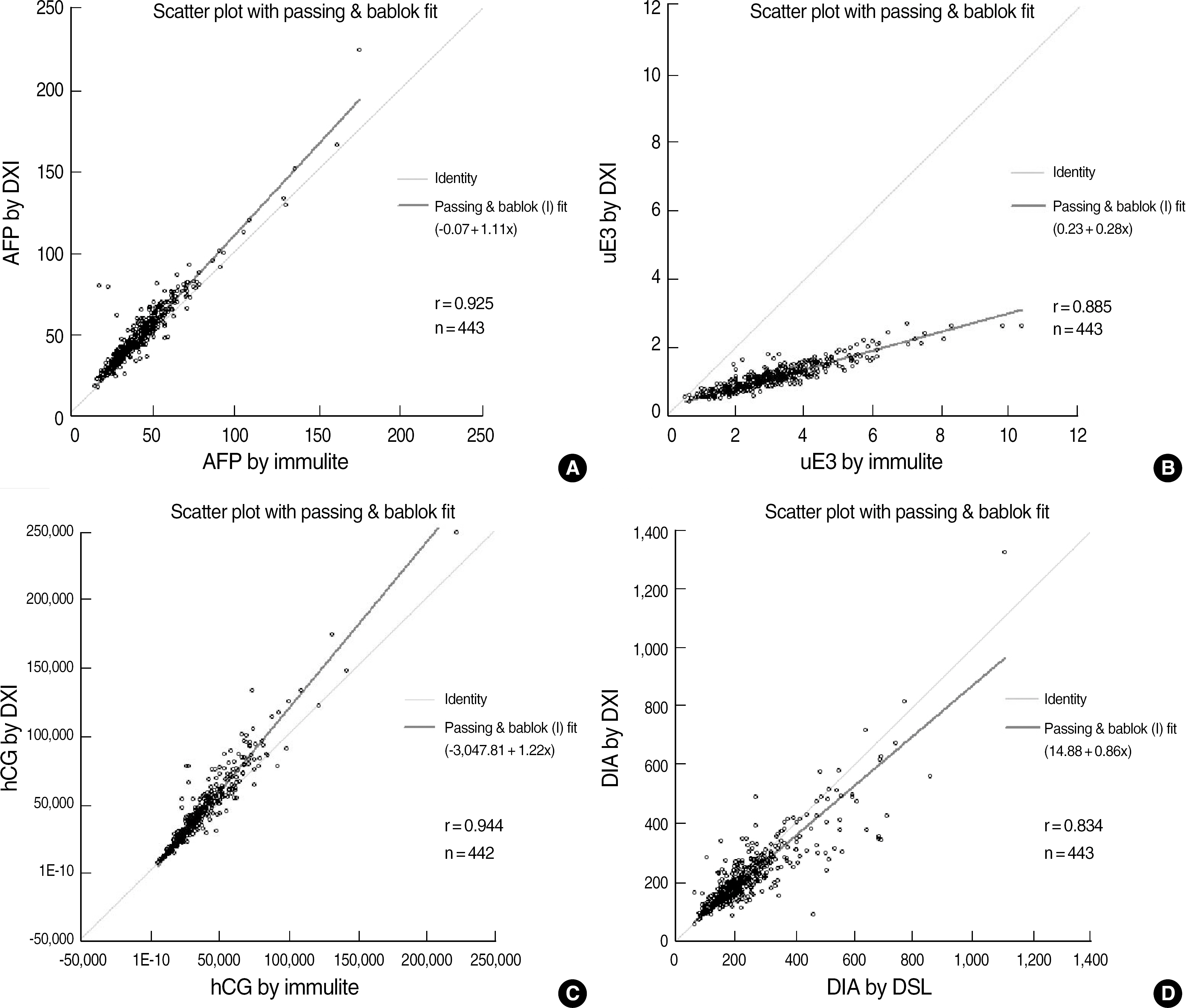
We measured the concentrations of each quadruple test analyte using the UniCel DxI 800 system (Beckman Coulter, USA) in 788 Korean mid-trimester maternal serum samples and calculated their median values using Benetech software (Benetech, Canada). We also compared the results with those obtained using the Immulite 2000 assay (Siemens Healthcare Diagnostics, USA) or ELISA (DSL, USA) in 442 samples.
RESULTS
We obtained mid-trimester median values for each marker. The following are the comparative results for each test using the Immulite 2000 assay or ELISA (x) and the UniCel DxI 800 immunoassay (y): AFP, y=1.10x+0.01, r=0.925; uE3, y=0.28x+0.24, r=0.885; hCG, y=1.22x-3047.8, r=0.944; and DIA, y=0.86x+15.31, r=0.833. Assay results for each of the four markers showed good correlations. However, significant biases necessitated new median calculations of prenatal risk estimates in all four tests.
CONCLUSIONS
We established gestational age-specific second-trimester median values for four markers in Korean samples using the UniCel DxI 800 immunoassay system. Despite significant bias, there were good correlations between the results obtained using the UniCel DxI 800 immunoassay and those obtained using the Immulite 2000 assay.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Evaluation of the UniCel™ DxI 800 Immunoassay Analyzer in Measuring Five Tumor Markers
Younhee Park, Yongjung Park, Jungyong Park, Hyon-Suk Kim
Yonsei Med J. 2012;53(3):557-564. doi: 10.3349/ymj.2012.53.3.557.
Reference
-
1.Wald NJ., Cuckle HS., Densem JW., Nanchahal K., Royston P., Chard T, et al. Maternal serum screening for Down's syndrome in early pregnancy. BMJ. 1988. 297:883–7.
Article2.Egan JF., Kaminsky LM., DeRoche ME., Barsoom MJ., Borgida AF., Benn PA. Antenatal Down syndrome screening in the United States in 2001: a survey of maternal-fetal medicine specialists. Am J Obstet Gynecol. 2002. 187:1230–4.
Article3.Kim JM., Sim AS., Lee EH. Amniotic chromosomal analysis in pregnant women identified by triple-marker testing as screen positive. Korean J Lab Med. 2006. 26:123–30.
Article4.Kim SK., Bai SW., Chung JE., Jung YN., Park KH., Cho DJ, et al. Triple marker screening for fetal chromosomal abnormalities in Korean women of advanced maternal age. Yonsei Med J. 2001. 42:199–203.
Article5.Han SH., An JW., Jeong GY., Yoon HR., Lee A., Yang YH, et al. Clinical and cytogenetic findings on 31,615 mid-trimester amniocenteses. Korean J Lab Med. 2008. 28:378–85.
Article6.Benn PA., Kaminsky LM., Ying J., Borgida AF., Egan JF. Combined second-trimester biochemical and ultrasound screening for Down syndrome. Obstet Gynecol. 2002. 100:1168–76.
Article7.Benn PA., Fang M., Egan JF., Horne D., Collins R. Incorporation of inhibin-A in second-trimester screening for Down syndrome. Obstet Gynecol. 2003. 101:451–4.
Article8.Wald NJ., Rodeck C., Hackshaw AK., Rudnicka A. SURUSS in perspective. Semin Perinatol. 2005. 29:225–35.
Article9.Lambert-Messerlian GM., Palomaki GE., Canick JA. Inhibin A measurement using an automated assay platform. Prenat Diagn. 2008. 28:399–403.
Article10.National Committee for Clinical Laboratory Standards. Evaluation of precision performance of quantitative measurement methods: approved guideline. NCCLS document EP5-A2. 2nd ed.Wayne, PA: NCCLS;2004.11.Rawlins ML., La'ulu SL., Erickson JA., Roberts WL. Performance characteristics of the Access Inhibin A assay. Clin Chim Acta. 2008. 397:32–5.
Article12.Erickson JA., Ashwood ER., Gin CA. Evaluation of a dimeric inhibin-A assay for assessing fetal Down syndrome: establishment, comparison, and monitoring of median concentrations for normal pregnancies. Arch Pathol Lab Med. 2004. 128:415–20.
Article13.Vranken G., Reynolds T., Van Nueten J. Medians for second-trimester maternal serum markers: geographical differences and variation caused by median multiples-of-median equations. J Clin Pathol. 2006. 59:639–44.
Article14.Crandall BF., Lebherz TB., Schroth PC., Matsumoto M. Alpha-fetoprotein concentrations in maternal serum: relation to race and body weight. Clin Chem. 1983. 29:531–3.
Article15.Bogart MH., Jones OW., Felder RA., Best RG., Bradley L., Butts W, et al. Prospective evaluation of maternal serum human chorionic gonadotropin levels in 3428 pregnancies. Am J Obstet Gynecol. 1991. 165:663–7.
Article16.Simpson JL., Elias S., Morgan CD., Shulman L., Umstot E., Andersen RN. Second trimester maternal serum human chorionic gonadotropin and unconjugated oestriol levels in blacks and whites. Lancet. 1990. 335:1459–60.
Article17.Benn PA., Clive JM., Collins R. Medians for second-trimester maternal serum alpha-fetoprotein, human chorionic gonadotropin, and unconjugated estriol; differences between races or ethnic groups. Clin Chem. 1997. 43:333–7.18.Benn PA., Collins R. Evaluation of effect of analytical imprecision in maternal serum screening for Down's syndrome. Ann Clin Biochem. 2001. 38:28–36.19.Serdar MA., Tutuncu L., Olgun A., Hasimi A., Ozgurtas T., Erbil MK. The effects of analytical factors on second trimester risk estimations. Int J Gynaecol Obstet. 2006. 93:28–32.
Article20.Wald NJ., Bestwick JP., Huttly WJ., Morris JK., George LM. Validation plots in antenatal screening for Down's syndrome. J Med Screen. 2006. 13:166–71.
Article21.Kim S., Kim YH., Min WK. Prenatal serum marker screening in Korea: survey results. Korean J Lab Med. 2007. 27:28–33.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Performance Evaluation of the Total Prostate- Specific Antigen (PSA), Free PSA, and [–2]proPSA Assay for Calculation of the Prostate Health Index Using the UniCel DxI 800 Analyzer
- Evaluation of the UniCel(TM) DxI 800 Immunoassay Analyzer in Measuring Five Tumor Markers
- Alpha-Fetoprotein Values in Maternal Serum and Amniotic Fluid for Prenatal Screening of Genetic Disorders
- Korean-Specific Parameter Models for Calculating the Risk of Down Syndrome in the Second Trimester of Pregnancy
- Performance Evaluation of the Serum Thyroglobulin Assays With Immunochemiluminometric Assay and Immunoradiometric Assay for Differentiated Thyroid Cancer