Yonsei Med J.
2012 May;53(3):557-564. 10.3349/ymj.2012.53.3.557.
Evaluation of the UniCel(TM) DxI 800 Immunoassay Analyzer in Measuring Five Tumor Markers
- Affiliations
-
- 1Department of Laboratory Medicine, Kwandong University College of Medicine, Goyang, Korea.
- 2Department of Laboratory Medicine, Yonsei University College of Medicine, Seoul, Korea. kimhs54@yuhs.ac
- KMID: 1776991
- DOI: http://doi.org/10.3349/ymj.2012.53.3.557
Abstract
- PURPOSE
Tumor marker concentrations in a given specimen measured by different analyzers vary according to assay methods, epitopes for antibodies used, and reagent specificities. Although great effort in quality assessment has been instituted, discrepancies among results from different analyzers are still present. We evaluated the assay performance of the UniCel(TM) DxI 800 automated analyzer in measuring the alpha-fetoprotein (AFP), carcinoembryonic antigen (CEA), carbohydrate antigen (CA) 125, CA 15-3 and CA 19-9 tumor markers.
MATERIALS AND METHODS
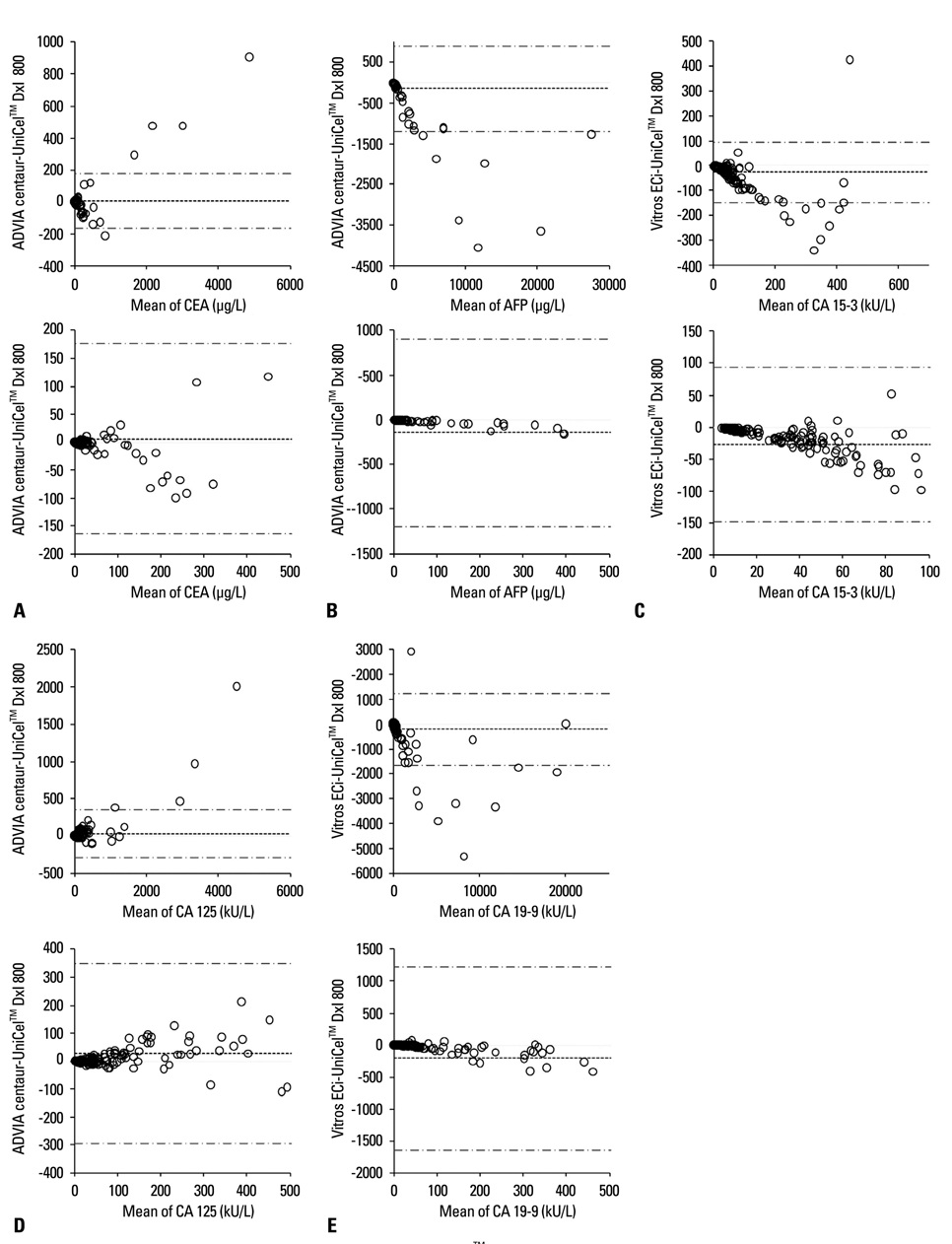
The linearity and precision performance of the five tumor marker assays were evaluated, and concentrations of the respective markers as measured by DxI were compared to those measured by other conventional analyzers (ADVIA Centaur(TM) and Vitros(TM) ECi) using 200 specimens collected from 100 healthy persons and 100 patients with respective cancers.
RESULTS
The linear fits for all five tumor markers were statistically acceptable (F=4648 for AFP, F=15846 for CEA, F=6445 for CA 125, F=2285 for CA 15-3, F=7459 for CA 19-9; p<0.0001 for all). The imprecision of each tumor marker assay was less than 5% coefficient of variation, except for low and high concentrations of AFP. The results from UniCel(TM) DxI 800 were highly correlated with those from other analyzers.
CONCLUSION
Our results demonstrate that UniCel(TM) DxI 800 has good linearity and precision performance for the tumor markers assayed in this study. However, there were discrepancies between assaying methods. Efforts to standardize tumor marker assays should be undertaken, and the redetermination of cut-off levels is necessary when developing methods of analyzing tumor markers.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Performance Evaluation and Clinical Usefulness of α-fetoprotein Test Measured on Sysmex HISCL-5000
Jaewan Jung, Eun-Suk Kang, Hyung-Doo Park
Lab Med Online. 2020;10(1):33-38. doi: 10.3343/lmo.2020.10.1.33.
Reference
-
1. Burtis CA, Ashwood ER, Bruns DE. Tietz textbook of clinical chemistry and molecular diagnostics. 2006. 4th ed. St. Louis: Elsevier saunders.2. Duffy MJ. Serum tumor markers in breast cancer: are they of clinical value? Clin Chem. 2006. 52:345–351.
Article3. Cheli CD, Morris DL, Neaman IE, Dai J, Allard WJ, Yeung KK. Measurement of four tumor marker antigens in the sera of pregnant women. J Clin Lab Anal. 1999. 13:35–39.
Article4. Connor S, Bosonnet L, Alexakis N, Raraty M, Ghaneh P, Sutton R, et al. Serum CA19-9 measurement increases the effectiveness of staging laparoscopy in patients with suspected pancreatic malignancy. Dig Surg. 2005. 22:80–85.
Article5. Bendardaf R, Lamlum H, Pyrhönen S. Prognostic and predictive molecular markers in colorectal carcinoma. Anticancer Res. 2004. 24:2519–2530.6. Ishigami S, Natsugoe S, Nakashima H, Tokuda K, Nakajo A, Okumura H, et al. Biological aggressiveness of alpha-fetoprotein (AFP)-positive gastric cancer. Hepatogastroenterology. 2006. 53:338–341.7. Toyoda H, Kumada T, Kiriyama S, Sone Y, Tanikawa M, Hisanaga Y, et al. Prognostic significance of simultaneous measurement of three tumor markers in patients with hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2006. 4:111–117.
Article8. Delwiche R, Zamcheck N, Marcon N. Carcinoembryonic antigen in pancreatitis. Cancer. 1973. 31:328–330.
Article9. Loewenstein MS, Zamcheck N. Carcinoembryonic antigen (CEA) levels in benign gastrointestinal disease states. Cancer. 1978. 42:3 Suppl. 1412–1418.
Article10. Gebauer G, Müller-Ruchholtz W. Carcinoembryonic antigen and CA19-9: implications of quantitative marker measurement in tissues for prognosis of colorectal cancer. Cancer Detect Prev. 2001. 25:344–351.11. Dungchai W, Siangproh W, Lin JM, Chailapakul O, Lin S, Ying X. Development of a sensitive micro-magnetic chemiluminescence enzyme immunoassay for the determination of carcinoembryonic antigen. Anal Bioanal Chem. 2007. 387:1965–1971.
Article12. Slev PR, Rawlins ML, Roberts WL. Performance characteristics of seven automated CA 15-3 assays. Am J Clin Pathol. 2006. 125:752–757.
Article13. Crombach G, Zippel HH, Würz H. Clinical significance of cancer antigen 125 (CA 125) in ovarian cancer. Cancer Detect Prev. 1985. 8:135–139.14. Yagmur E, Driesch R, Gressner AM, Kiefer P. Technical evaluation of the Beckman Coulter OV-Monitor (CA 125 antigen) immunoassay. Clin Chem Lab Med. 2006. 44:420–422.
Article15. Deinzer M, Faissner R, Metzger T, Kaminski WE, Löhr M, Neumaier M, et al. Comparison of two different methods for CA19-9 antigen determination. Clin Lab. 2010. 56:319–325.16. Lee JH, Park Y, Suh B, Song SM, Kwon OH, Kim JH. Performance characteristics of the UniCel DxI 800 immunoassay for the maternal serum quadruple test, including median values for each week of gestation, in Korean women. Korean J Lab Med. 2010. 30:126–132.
Article17. Passing H, Bablok . A new biometrical procedure for testing the equality of measurements from two different analytical methods. Application of linear regression procedures for method comparison studies in clinical chemistry, Part I. J Clin Chem Clin Biochem. 1983. 21:709–720.
Article18. Passing H, Bablok W. Comparison of several regression procedures for method comparison studies and determination of sample sizes. Application of linear regression procedures for method comparison studies in Clinical Chemistry, Part II. J Clin Chem Clin Biochem. 1984. 22:431–445.
Article19. Bland JM, Altman DG. Comparing methods of measurement: why plotting difference against standard method is misleading. Lancet. 1995. 346:1085–1087.
Article20. Nicolini A, Carpi A, Michelassi C, Spinelli C, Conte M, Miccoli P, et al. "Tumour marker guided" salvage treatment prolongs survival of breast cancer patients: final report of a 7-year study. Biomed Pharmacother. 2003. 57:452–459.
Article21. Mongia SK, Rawlins ML, Owen WE, Roberts WL. Performance characteristics of seven automated CA 125 assays. Am J Clin Pathol. 2006. 125:921–927.
Article22. Stern P, Friedecky B, Bartos V, Bezdickova D, Vavrova J, Uhrova J, et al. Comparison of different immunoassays for CA 19-9. Clin Chem Lab Med. 2001. 39:1278–1282.
Article23. Ognibene A, Drake CJ, Jeng KY, Pascucci TE, Hsu S, Luceri F, et al. A new modular chemiluminescence immunoassay analyser evaluated. Clin Chem Lab Med. 2000. 38:251–260.
Article24. Liew M, Groll MC, Thompson JE, Call SL, Moser JE, Hoopes JD, et al. Validating a custom multiplex ELISA against individual commercial immunoassays using clinical samples. Biotechniques. 2007. 42:327–328. 330–333.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Performance Evaluation of the Total Prostate- Specific Antigen (PSA), Free PSA, and [–2]proPSA Assay for Calculation of the Prostate Health Index Using the UniCel DxI 800 Analyzer
- Performance Characteristics of the UniCel DxI 800 Immunoassay for the Maternal Serum Quadruple Test, Including Median Values for Each Week of Gestation, in Korean Women
- Analytical and Clinical Assessment of Prostate Specific Antigen Using an HISCL-5000 Chemiluminescence Immunoassay
- Performance Evaluation of the Serum Thyroglobulin Assays With Immunochemiluminometric Assay and Immunoradiometric Assay for Differentiated Thyroid Cancer
- Performance Evaluation of Body Fluid Cellular Analysis Using the Beckman Coulter UniCel DxH 800, Sysmex XN-350, and UF-5000 Automated Cellular Analyzers