J Korean Med Sci.
2005 Apr;20(2):248-255. 10.3346/jkms.2005.20.2.248.
Angiotensin II AT1 Receptor Blockade Changes Expression of Renal Sodium Transporters in Rats with Chronic Renal Failure
- Affiliations
-
- 1Department of Physiology, School of Medicine, Dongguk University, Kyungju, Korea.
- 2Department of Anatomy, School of Medicine, Dongguk University, Kyungju, Korea.
- 3Department of Biochemistry and Cell Biology, School of Medicine, Kyungpook National University, Daegu, Korea. thkwon@knu.ac.kr
- KMID: 1095199
- DOI: http://doi.org/10.3346/jkms.2005.20.2.248
Abstract
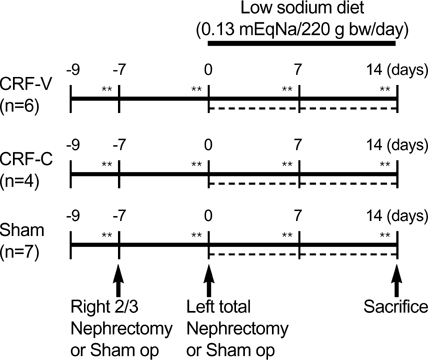
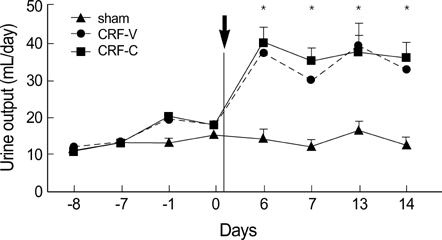
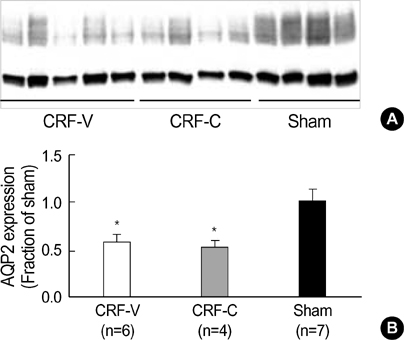
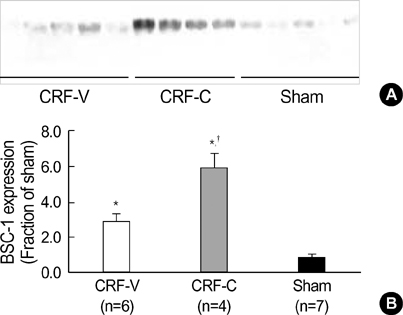
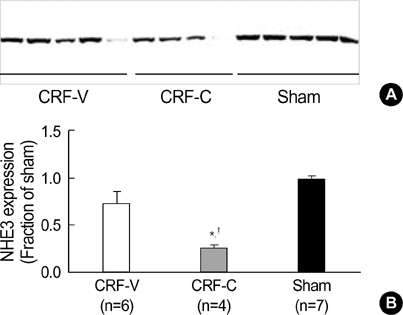
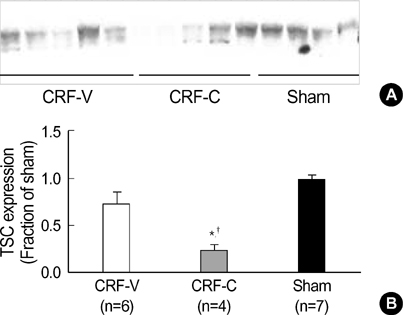
- We aimed to examine the effects of angiotensin II AT1 receptor blocker on the expression of major renal sodium transporters and aquaporin-2 (AQP2) in rats with chronic renal failure (CRF). During 2 wks after 5/6 nephrectomy or sham operation, both CRF rats (n=10) and sham-operated control rats (n=7) received a fixed amount of low sodium diet and had free access to water. CRF rats (n=10) were divided into two groups which were either candesartan-treated (CRF-C, n=4) or vehicletreated (CRF-V, n=6). Both CRF-C and CRF-V demonstrated azotemia, decreased GFR, polyuria, and decreased urine osmolality compared with sham-operated rats. When compared with CRF-V, CRF-C was associated with significantly higher BUN levels and lower remnant kidney weight. Semiquantitative immunoblotting demonstrated decreased AQP2 expression in both CRF-C (54% of control levels) and CRF-V (57%), whereas BSC-1 expression was increased in both CRF groups. Particularly, CRF-C was associated with higher BSC-1 expression (611%) compared with CRF-V (289%). In contrast, the expression of NHE3 (25%) and TSC (27%) was decreased in CRF-C, whereas no changes were observed in CRF-V. In conclusion, 1) candesartan treatment in an early phase of CRF is associated with decreased renal hypertrophy and increased BUN level; 2) decreased AQP2 level in CRF is likely to play a role in the decreased urine concentration, and the downregulation is not altered in response to candesartan treatment; 3) candesartan treatment decreases NHE3 and TSC expression; and 4) an increase of BSC-1 is prominent in candesartan-treated CRF rats, which could be associated with the increased delivery of sodium and water to the thick ascending limb.
MeSH Terms
-
Angiotensin II Type 1 Receptor Blockers
Animals
Aquaporins/genetics
Benzimidazoles/*pharmacology
Blood Urea Nitrogen
Kidney Failure, Chronic/drug therapy/*metabolism
Male
Organ Size/drug effects
Rats
Rats, Sprague-Dawley
Receptors, Drug/*genetics
Research Support, Non-U.S. Gov't
Sodium-Hydrogen Antiporter/*genetics
Sodium-Potassium-Chloride Symporters/*genetics
Symporters/*genetics
Tetrazoles/*pharmacology
Figure
Reference
-
1. Hayslett JP. Functional adaptation to reduction in renal mass. Physiol Rev. 1979. 59:137–164.
Article2. Slatopolsky E, Elkan IO, Weerts C, Bricker NS. Studies on the characteristics of the control system governing sodium excretion in uremic man. J Clin Invest. 1968. 47:521–530.
Article3. Hayslett JP, Kashgarian M, Epstein FH. Mechanism of change in the excretion of sodium per nephron when renal mass is reduced. J Clin Invest. 1969. 48:1002–1006.
Article4. Trizna W, Yanagawa N, Bar Khayim Y, Houston B, Fine LG. Functional profile of the isolated uremic nephron. Evidence of proximal tubular "memory" in experimental renal disease. J Clin Invest. 1981. 68:760–767.5. Fine LG, Trizna W, Bourgoignie JJ, Bricker NS. Functional profile of the isolated uremic nephron. Role of compensatory hypertrophy in the control of fluid reabsorption by the proximal straight tubule. J Clin Invest. 1978. 61:1508–1518.6. Kwon TH, Frokiaer J, Fernandez-Llama P, Maunsbach AB, Knepper MA, Nielsen S. Altered expression of Na transporters NHE-3, NaPi-II, Na-K-ATPase, BSC-1, and TSC in CRF rat kidneys. Am J Physiol. 1999. 277:257–270.7. Kwon TH, Frokiaer J, Knepper MA, Nielsen S. Reduced AQP1, -2, and -3 levels in kidneys of rats with CRF induced by surgical reduction in renal mass. Am J Physiol. 1998. 275:724–741.8. Eliahou H, Avinoach I, Shahmurov M, Ben David A, Shahar C, Matas Z, Zimlichman R. Renoprotective effect of angiotensin II receptor antagonists in experimental chronic renal failure. Am J Nephrol. 2001. 21:78–83.
Article9. Uhlenius N, Miettinen A, Vuolteenaho O, Tikkanen I. Renoprotective mechanisms of angiotensin II antagonism in experimental chronic renal failure. Kidney Blood Press Res. 2002. 25:71–79.
Article10. Kwon TH, Nielsen J, Kim YH, Knepper MA, Frokiaer J, Nielsen S. Regulation of sodium transporters in the thick ascending limb of rat kidney: response to angiotensin II. Am J Physiol Renal Physiol. 2003. 285:152–165.11. Kwon TH, Nielsen J, Masilamani S, Hager H, Knepper MA, Frokiaer J, Nielsen S. Regulation of collecting duct AQP3 expression: response to mineralocorticoid. Am J Physiol Renal Physiol. 2002. 283:1403–1421.12. Nielsen J, Kwon TH, Masilamani S, Beutler K, Hager H, Nielsen S, Knepper MA. Sodium transporter abundance profiling in kidney: effect of spironolactone. Am J Physiol Renal Physiol. 2002. 283:923–933.13. Kim GH, Masilamani S, Turner R, Mitchell C, Wade JB, Knepper MA. The thiazide-sensitive Na-Cl cotransporter is an aldosterone-induced protein. Proc Natl Acad Sci USA. 1998. 95:14552–14557.
Article14. Beutler KT, Masilamani S, Turban S, Nielsen J, Brooks HL, Ageloff S, Fenton RA, Packer RK, Knepper MA. Long-term regulation of ENaC expression in kidney by angiotensin II. Hypertension. 2003. 41:1143–1150.
Article15. Inscho EW, Imig JD, Deichmann PC, Cook AK. Candesartan cilexetil protects against loss of autoregulatory efficiency in angiotensin II-infused rats. J Am Soc Nephrol. 1999. 10:Suppl 11. S178–S183.16. Guo DF, Tardif V, Ghelima K, Chan JS, Ingelfinger JR, Chen X, Chenier I. A novel angiotensin II type 1 receptor-associated protein induces cellular hypertrophy in rat vascular smooth muscle and renal proximal tubular cells. J Biol Chem. 2004. 279:21109–21120.
Article17. Mazzolai L, Pedrazzini T, Nicoud F, Gabbiani G, Brunner HR, Nussberger J. Increased cardiac angiotensin II levels induce right and left ventricular hypertrophy in normotensive mice. Hypertension. 2000. 35:985–991.
Article18. Knepper MA, Nielsen S, Chou CL, DiGiovanni SR. Mechanism of vasopressin action in the renal collecting duct. Semin Nephrol. 1994. 14:302–321.19. Stephenson JL. Countercurrent transport in the kidney. Annu Rev Biophys Bioeng. 1978. 7:315–339.
Article20. Kwon TH, Hager H, Nejsum LN, Andersen ML, Frokiaer J, Nielsen S. Physiology and pathophysiology of renal aquaporins. Semin Nephrol. 2001. 21:231–238.
Article21. Buerkert J, Martin D, Prasad J, Chambless S, Klahr S. Response of deep nephrons and the terminal collecting duct to a reduction in renal mass. Am J Physiol. 1979. 236:454–464.
Article22. Tannen RL, Regal EM, Dunn MJ, Schrier RW. Vasopressin-resistant hyposthenuria in advanced chronic renal disease. N Engl J Med. 1969. 280:1135–1141.
Article23. Fine LG, Schlondorff D, Trizna W, Gilbert RM, Bricker NS. Functional profile of the isolated uremic nephron. Impaired water permeability and adenylate cyclase responsiveness of the cortical collecting tubule to vasopressin. J Clin Invest. 1978. 61:1519–1527.24. Teitelbaum I, McGuinness S. Vasopressin resistance in chronic renal failure. Evidence for the role of decreased V2 receptor mRNA. J Clin Invest. 1995. 96:378–385.
Article25. Kwon TH, Nielsen J, Knepper MA, Frokiaer J, Nielsen S. Angiotensin II AT1 receptor blockade decreases vasopressin-induced water reabsorption and AQP2 levels in Nacl-restricted rats. Am J physiol Renal Physiol. 2005. 288:673–683.
Article26. Hus-Citharel A, Marchetti J, Corvol P, Llorens-Cortes C. Potentiation of [Ca2+]i response to angiotensin III by cAMP in cortical thick ascending limb. Kidney Int. 2002. 61:1996–2005.
Article27. Klingler C, Ancellin N, Barrault MB, Morel A, Buhler JM, Elalouf JM, Clauser E, Lugnier C, Corman B. Angiotensin II potentiates vasopressin-dependent cAMP accumulation in CHO transfected cells. Mechanisms of cross-talk between AT1A and V2 receptors. Cell Signal. 1998. 10:65–74.
Article28. Knepper MA, Kim GH, Fernandez-Llama P, Ecelbarger CA. Regulation of thick ascending limb transport by vasopressin. J Am Soc Nephrol. 1999. 10:628–634.
Article29. Wang W, Li C, Kwon TH, Miller RT, Knepper MA, Frokiaer J, Nielsen S. Reduced expression of renal Na+ transporters in rats with PTH-induced hypercalcemia. Am J Physiol Renal Physiol. 2004. 286:534–545.
Article30. Elkjaer ML, Kwon TH, Wang W, Nielsen J, Knepper MA, Frokiaer J, Nielsen S. Altered expression of renal NHE3, TSC, BSC-1, and ENaC subunits in potassium-depleted rats. Am J Physiol Renal Physiol. 2002. 283:1376–1388.31. Ecelbarger CA, Terris J, Hoyer JR, Nielsen S, Wade JB, Knepper MA. Localization and regulation of the rat renal Na(+)-K(+)-2Cl-cotransporter, BSC-1. Am J Physiol. 1996. 271:619–628.32. Landwehr DM, Klose RM, Giebisch G. Renal tubular sodium and water reabsorption in the isotonic sodium chloride-loaded rat. Am J Physiol. 1967. 212:1327–1333.
Article33. Brooks HL, Sorensen AM, Terris J, Schultheis PJ, Lorenz JN, Shull GE, Knepper MA. Profiling of renal tubule Na+ transporter abundances in NHE3 and NCC null mice using targeted proteomics. J Physiol. 2001. 530:359–366.34. Lorenz JN, Schultheis PJ, Traynor T, Shull GE, Schnermann J. Micropuncture analysis of single-nephron function in NHE3-deficient mice. Am J Physiol. 1999. 277:447–453.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- ACE2 and Angiotensin-(1-7) in Hypertensive Renal Disease
- Renal Interstitial Fibrosis and Angiotensin Inhibition
- Effect of brain angiotensin II receptor antagonists and antisense oligonucleotide on drinking and renal renin in rats
- Altered Regulation of Renal Aquaporins and Sodium Transporters in Experimental Chronic Renal Failure
- Angiotensin converting enqyme inhibitor and angiotensin II AT1 receptor antagonist in progressive renal disease