J Vet Sci.
2007 Sep;8(3):263-267. 10.4142/jvs.2007.8.3.263.
Bioavailability of the amino acid-attached prodrug as a new anti-HIV agent in rats
- Affiliations
-
- 1Department of Veterinary Pharmacology and Toxicology, College of Veterinary Medicine, Konkuk University, Seoul 143-701, Korea. hshin@konkuk.ac.kr
- KMID: 1090801
- DOI: http://doi.org/10.4142/jvs.2007.8.3.263
Abstract
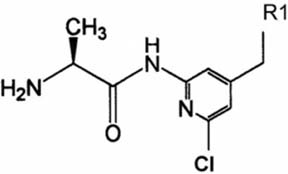
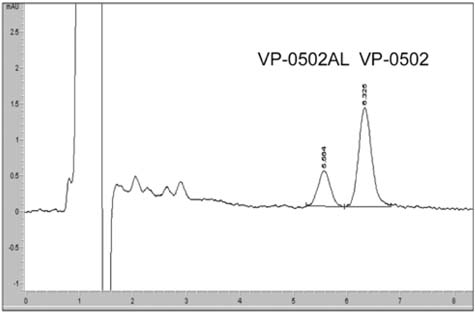
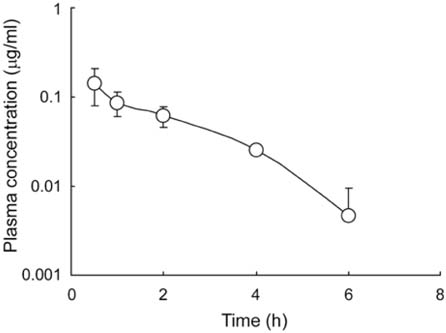
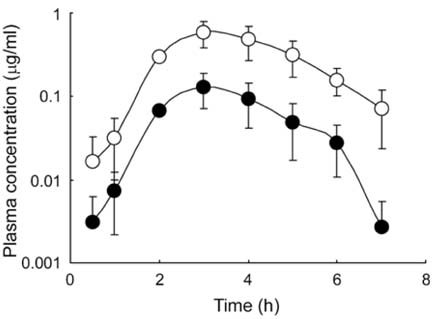
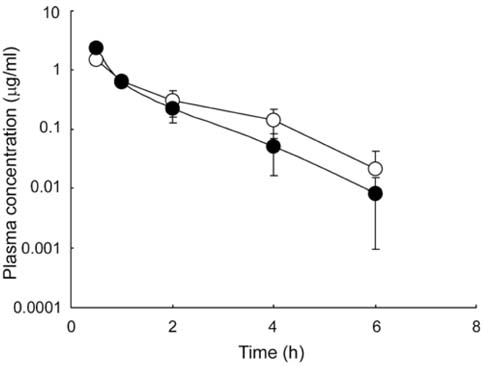
- The primary objective of this study was to compare thepharmacokinetics of a new anti-human immunodeficiencyvirus agent 1-(2-amino-pyridin-4-ylmethyl)-6-(3,5-dimethyl-benzoyl)-5-isopropyl-1H-pyrimidine-2,4-dione (VP-0502)with its amino acid prodrug alanine amide of VP-0502(VP-0502AL), following intravenous and oral administrationsto rats. The plasma concentrations of both analytes wereanalyzed via high-performance liquid chromatographycoupled with photodiode-array detection (HPLC-DAD).When VP-0502 was intravenously administered at 20mg/kg, the analyte appeared in low levels with an AUC of 0.3microg.h/ml, and C0 of 0.2microg/ml in plasma. However, boththe prodrug VP-0502AL and its metabolite VP-0502 appearedat comparatively higher levels following intravenousinjection of VP-0502AL at the same dose. VP-0502AL'spharmacokinetic parameters were Vd: 4.6 l/kg; AUC:3microg.h/ml; t1/2: 0.5h; C0: 6microg/ml; CLtot: 7l/h/kg; andMRT: 0.6h. Following oral administration of VP-0502(100mg/kg), it was not detectable in plasma (<50ng/ml),while after the oral administration of VP-0502AL, VP-0502 was quantitatively detected as an active metabolite forthe first 7h, with a maximum plasma concentration(Cmax) of 0.8microg/ml, and an area under the concentration-time curve (AUC) of 2microg.h/ml. The oral pharmacokineticparameters of VP-0502AL were calculated to be: maximumconcentration time (tmax) 2.7h; Cmax 0.2microg/ml; eliminationhalf-life (t1/2): 0.8h; and AUC 0.5microg.h/ml. Overall thefindings indicate that VP-0502AL has a favorable pharmaco-kinetic profile as a prodrug with rapid transformationinto the active metabolite, and that the attachment of theamino acid alanine to VP-0502 is an effective approach toimprove its oral bioavailability. VP-0502AL is predictedto become a new highly bioavailable anti-AIDS drugcandidate and/or lead compound.
Keyword
MeSH Terms
-
Administration, Oral
Alanine/*analogs & derivatives/pharmacokinetics
Aminopyridines/*pharmacokinetics
Animals
Anti-HIV Agents/administration & dosage/blood/*pharmacokinetics
Area Under Curve
Biological Availability
Half-Life
Injections, Intravenous
Male
Prodrugs/administration & dosage/*pharmacokinetics
Rats
Rats, Sprague-Dawley
Uracil/*analogs & derivatives/pharmacokinetics
Figure
Reference
-
1. Abe Y, Shibata H, Kamada H, Tsunoda S, Tsutsumi Y, Nakagawa S. Promotion of optimized protein therapy by bioconjugation as a polymeric DDS. Anticancer Agents Med Chem. 2006. 6:251–258.
Article2. Arnold E, Das K, Ding J, Yadav PN, Hsiou Y, Boyer PL, Hughes SH. Targeting HIV reverse transcriptase for anti-AIDS drug design: structual and biological consideration for chemotherapeutic strategies. Drug Des Discov. 1996. 13:29–47.3. Bardsley-Elliot A, Perry CM. Nevirapine: a review of its use in the prevention and treatment of paediatric HIV infection. Paediatr Drugs. 2000. 2:373–407.4. Cho HJ, Choi KA, Sung JM, Jeong SM, Han JS, Kim JS, Shin HC. Pharmacokinetics of a new anti-HIV agent VP-0501 and development of its amino acid prodrug for improving oral bioavailability. Korean J Vet Res. 2006. 46:7–12.5. Dannenfelser RM, He H, Joshi Y, Bateman S, Serajuddin AT. Development of clinical dosage forms for a poorly water soluble drug I: Application of polyethylene glycol-polysorbate 80 solid dispersion carrier system. J Pharm Sci. 2004. 93:1165–1175.
Article6. Kondo N, Iwao T, Hirai K, Fukuda M, Yamanouchi K, Yokoyama K, Miyaji M, Ishihara Y, Kon K, Ogawa Y, Mayumi T. Improved oral absorption of enteric coprecipitates of a poorly soluble drug. J Pharm Sci. 1994. 83:566–570.
Article7. Kuksal A, Tiwary AK, Jain NK, Jain S. Formulation and in vitro, in vivo evaluation of extended-release matrix tablet of zidovudine: influence of combination of hydrophilic and hydrophobic matrix formers. AAPS Pharm Sci Tech. 2006. 7:E1–E9.8. Lin JH, Storey DE, Chen IW, Xu X. Improved oral absorption of L-365; 260:a poorly soluble drug. Biopharm Drug Dispos. 1996. 17:1–15.9. Lorenzi PL, Landowski CP, Song X, Borysko KZ, Breitenbach JM, Kim JS, Hilfinger JM, Townsend LB, Drach JC, Amidon GL. Amino acid ester prodrugs of 2-bromo-5,6-dichloro-1-(β-D-ribofuranosyl) benzimidazole enhance metabolic stability in vitro and in vivo. J Pharmacol Exp Ther. 2005. 314:883–890.
Article10. Song X, Vig BS, Lorenzi PL, Drach JC, Townsend LB, Amidon GL. Amino acid ester prodrugs of the antiviral agent 2-bromo-5,6-dichloro-1-(β-D-ribofuranosyl) benzimidazole as potential substrates of hPEPT1 transporter. J Med Chem. 2005. 48:1274–1277.
Article11. Sridevi S, Chauhan AS, Chalasani KB, Jain AK, Diwan PV. Enhancement of dissolution and oral bioavailability of gliquidone with hydroxy propyl-beta-cyclodextrin. Pharmazie. 2003. 58:807–810.12. Sriram D, Yogeeswari P, Srichakravarthy N, Bal TR. Synthesis of stavudine amino acid ester prodrugs with broad-spectrum chemotherapeutic properties for the effective treatment of HIV/AIDS. Bioorg Med Chem Lett. 2004. 14:1085–1087.
Article13. Subramanian N, Ray S, Ghosal SK, Bhadra R, Moulik SP. Formulation design of self-microemulsifying drug delivery systems for improved oral bioavailability of celecoxib. Biol Pharm Bull. 2004. 27:1993–1999.
Article14. Tantillo C, Ding J, Jacobo-Molina A, Nanni RG, Boyer PL, Hughes SH, Pauwels R, Andries K, Janssen PA, Arnold E. Locations of anti-AIDS drug binding sites and resistance mutations in the three-dimensional structure of HIV-1 reverse transcriptase. Implications for mechanisms of drug inhibition and resistance. J Mol Biol. 1994. 243:369–387.
Article15. Tansey W, Ke S, Cao XY, Pasuelo MJ, Wallace S, Li C. Synthesis and characterization of branched poly (L-glutamic acid) as a biodegradable drug carrier. J Control Release. 2004. 94:39–51.
Article16. Vig BS, Lorenzi PJ, Mittal S, Landowski CP, Shin HC, Mosberg HI, Hilfinger JM, Amidon GL. Amino acid ester prodrugs of floxuridine: synthesis and effects of structure, stereochemistry, and site of esterification on the rate of hydrolysis. Pharm Res. 2003. 20:1381–1388.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Analysis of Viral Phenotype (SI / NSI) and V3 Domain Amino Acid Sequence in the Various HIV - 1 Subtype Isolates
- Experience of the Use of Three Screening Kits, Enzygnost Anti-HIV1/2 Plus, ABBOTT TESTPACK HIV- 1/HIV-2 & SERODIA. HIV- 1/2 for the Detection of Antibodies to HIV
- Amino Acid Transporters as Potential Therapeutic Targets in Thyroid Cancer
- New Development of Anti-HIV Drugs
- Application of HIV-1 Complementation System to Screen the Anti-AIDS Agents That Targets the Late Stage of HIV-1 Replication Cycle