Yonsei Med J.
2010 May;51(3):385-391. 10.3349/ymj.2010.51.3.385.
Aldose Reductase Inhibitor Ameliorates Renal Vascular Endothelial Growth Factor Expression in Streptozotocin-Induced Diabetic Rats
- Affiliations
-
- 1Department of Internal Medicine, Yonsei University Wonju College of Medicine, Wonju, Korea. cchung@yonsei.ac.kr
- 2Center for Health Promotion, Samsung Medical Center, Sungkyunkwan University, Seoul, Korea.
- 3Department of Internal Medicine, Sun General Hospital, Daejeon, Korea.
- 4Department of Pathology, Yonsei University College of Medicine, Seoul, Korea.
- 5Department of Internal Medicine, Soonchunhyang University College of Medicine, Cheonan, Korea.
- KMID: 1074990
- DOI: http://doi.org/10.3349/ymj.2010.51.3.385
Abstract
- PURPOSE
The vascular endothelial growth factor (VEGF) expression of podocyte is one of the well-known major factors in development of diabetic nephropathy. In this study, we investigated the effects of aldose reductase inhibitor, fidarestat on diabetic nephropathy, and renal VEGF expression in a type 1 diabetic rat model.
MATERIALS AND METHODS
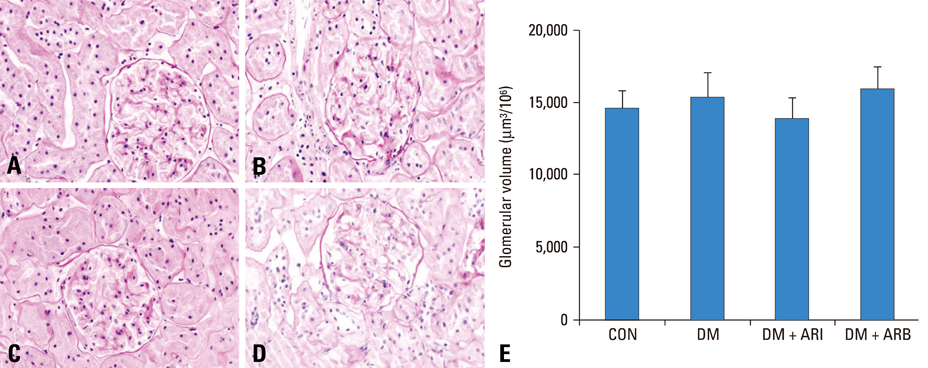
Twenty four Sprague-Dawley male rats which were performed intraperitoneal injection of streptozotocin and normal six rats were divided into four groups including a normal control group, untreated diabetic control group, aldose reductase (AR) inhibitor (fidarestat, 16 mg.kg(-1).day(-1)) treated diabetic group, and angiotensin receptor blocker (losartan, 20 mg.kg(-1).day(-1)) treated diabetic group. We checked body weights and blood glucose levels monthly and measured urine albumin-creatinine ratio (ACR) at 8 and 32 weeks. We extracted the kidney to examine the renal morphology and VEGF expressions.
RESULTS
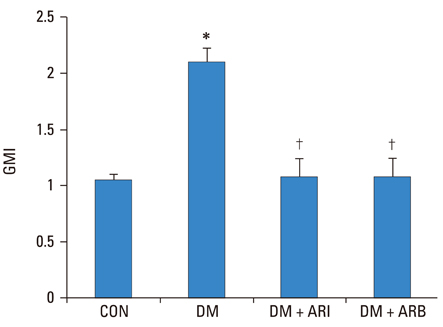
The ACR decreased in fidarestat and losartan treated diabetic rat groups than in untreated diabetic group (24.79 +/- 11.12, 16.11 +/- 9.95, and 84.85 +/- 91.19, p < 0.05). The renal VEGF messenger RNA (mRNA) and protein expression were significantly decreased in the fidarestat and losartan treated diabetic rat groups than in the diabetic control group.
CONCLUSION
We suggested that aldose reductase inhibitor may have preventive effect on diabetic nephropathy by reducing renal VEGF overexpression.
Keyword
MeSH Terms
-
Aldehyde Reductase/*antagonists & inhibitors
Animals
Antihypertensive Agents/therapeutic use
Diabetes Mellitus, Experimental/*drug therapy/*metabolism
Diabetic Nephropathies/prevention & control
Imidazolidines/*therapeutic use
Kidney/*drug effects/*metabolism/pathology
Losartan/therapeutic use
Male
Rats
Rats, Sprague-Dawley
Receptors, Angiotensin/*antagonists & inhibitors
Vascular Endothelial Growth Factor A
Figure
Reference
-
1. Derubertis FR, Craven PA. Activation of protein kinase C in glomerular cells in diabetes. Mechanisms and potential links to the pathogenesis of diabetic glomerulopathy. Diabetes. 1994. 43:1–8.
Article2. Yamagishi S, Inagaki Y, Okamoto Y, Amano S, Koga K, Takeeuchi M, et al. Advanced glycation end product-induced apoptosis and overexpression of vascular endothelial growth factor and monocyte chemoattractant protein-1 in human-cultured mesangial cells. J Biol Chem. 2002. 227:20309–20315.
Article3. Natarajan R, Bai W, Lanting L, Gonzales N, Nadler J. Effects of high glucose on vascular endothelial growth factor expression in vascular smooth muscle cells. Am J Physiol. 1997. 273:H2224–H2231.4. Keogh RJ, Dunlop ME, Larkins RG. Effect of inhibition of aldose reductase on glucose flux, diacylglycerol formation, protein kinase C, and phospholipase A2 activation. Metabolism. 1997. 46:41–47.5. Kim NH, Jung HH, Cha DR, Choi DS. Expression of vascular endothelial growth factor in response to high glucose in rat mesangial cells. J Endocrinol. 2000. 165:617–624.
Article6. Tsuchida K, Makita Z, Yamagishi S, Atsumi T, Miyoshi H, Obara S, et al. Suppression of transforming growth factor beta and vascular endothelial growth factor in diabetic nephropathy in rats by a novel advanced glycation end product inhibitor, OPB-9195. Diabetologia. 1999. 42:579–588.
Article7. Lu M, Kuroki M, Amano S, Tolentino M, Keough K, Kim I, et al. Advanced glycation end products increase retinal vascular endothelial growth factor expression. J Clin Invest. 1998. 101:1219–1224.
Article8. Wang L, Kwak JH, Kim SI, He Y, Choi ME. Transforming growth factor-beta1 stimulates vascular endothelial growth factor 164 via mitogen-activated protein kinase kinase 3-p38alpha and p38delta mitogen-activated protein kinase-dependent pathway in murine mesangial cells. J Biol Chem. 2004. 279:33213–33219.
Article9. Kuroki M, Voest EE, Amano S, Beerepoot LV, Takashima S, Tolentino M, et al. Reactive oxygen intermediates increase vascular endothelial growth factor expression in vitro and in vivo. J Clin Invest. 1996. 98:1667–1675.
Article10. Ferrara N. Role of vascular endothelial growth factor in the regulation of angiogenesis. Kidney Int. 1999. 56:794–814.
Article11. Cha DR, Kim IS, Kang YS, Han SY, Han KH, Shin C, et al. Urinary concentration of transforming growth factor-beta-inducible gene-h3(beta ig-h3) in patients with Type 2 diabetes mellitus. Diabet Med. 2005. 22:14–20.
Article12. Cooper ME, Vranes D, Youssef S, Stacker SA, Cox AJ, Rizkalla B, et al. Increased renal expression of vascular endothelial growth factor (VEGF) and its receptor VEGFR-2 in experimental diabetes. Diabetes. 1999. 48:2229–2239.13. de Vriese AS, Tilton RG, Elger M, Stephan CC, Kriz W, Lameire NH. Antibodies against vascular endothelial growth factor improve early renal dysfunction in experimental diabetes. J Am Soc Nephrol. 2001. 12:993–1000.
Article14. Lee EY, Shim MS, Kim MJ, Hong SY, Shin YG, Chung CH. Angiotensin II receptor blocker attenuates overexpression of vascular endothelial growth factor in diabetic podocytes. Exp Mol Med. 2004. 36:65–70.
Article15. Fonseca VA. Clinical diabetes: translating research into practice. 2006. Philadelphia: Saunders Elsevier.16. Tilton RG, Kawamura T, Chang KC, Ido Y, Bjercke RJ, Stephan CC, et al. Vascular dysfunction induced by elevated glucose levels in rats is mediated by vascular endothelial growth factor. J Clin Invest. 1997. 99:2192–2202.
Article17. Obrosova IG, Minchenko AG, Vasupuram R, White L, Abatan OI, Kumagai AK, et al. Aldose reductase inhibitor fidarestat prevents retinal oxidative stress and vascular endothelial growth factor overexpression in streptozotocin-diabetic rats. Diabetes. 2003. 52:864–871.
Article18. Lane PH, Steffes MW, Mauer SM. Estimation of glomerular volume: a comparison of four methods. Kidney Int. 1992. 41:1085–1089.19. Saito T, Sumithran E, Glasgow EF, Atkins RC. The enhancement of aminonucleoside nephrosis by the co-administration of protamine. Kidney Int. 1987. 32:691–699.20. Ziyadeh FN. The extracelluar matrix in diabetic nephropathy. Am J Kidney Dis. 1993. 22:736–744.21. Craven PA, DeRubertis FR. Protein kinase C is activated in glomeruli from streptozotocin diabetic rats. Possible mediation by glucose. J Clin Invest. 1989. 83:1667–1675.
Article22. Ghahary A, Luo JM, Gong YW, Chakrabarti S, Sima AA, Murphy LJ. Increased renal aldose reductase activity, immunoreactivity, and mRNA in streptozocin-induced diabetic rats. Diabetes. 1989. 38:1067–1071.
Article23. Raptis AE, Viberti G. Pathogenesis of diabetic nephropathy. Exp Clin Endocrinol Diabetes. 2001. 109:Suppl 2. S424–S437.
Article24. Donnelly SM, Zhou XP, Huang JT, Whiteside CI. Prevention of early glomerulopathy with tolrestat in the streptozotocin-induced diabetic rat. Biochem Cell Biol. 1996. 74:355–362.
Article25. Isogai S, Inokuchi T, Ohe K. Effect of an aldose reductase inhibitor on glomerular basement membrane anionic sites in streptozotocin-induced diabetic rats. Diabetes Res Clin Pract. 1995. 30:111–116.26. Obrosova IG. How dose glucose generate oxidative stress in peripheral nerve? Int Rev of Neurobiol. 2002. 50:3–35.27. Lee AY, Chung SS. Contributions of polyol pathway to oxidative stress in diabetic cataract. FASEB J. 1999. 13:23–30.
Article28. Obrosova IG, Van Huysen C, Fathallah L, Cao XC, Greene DA, Stevens MJ. An aldose reductase inhibitor reverse early diabetes-induced changes in peripheral nerve function, metabolism, and antioxidative defense. FASEB J. 2002. 16:123–125.29. Kuroki M, Voest EE, Amano S, Beerepoot LV, Takashima S, Tolentino M, et al. Reactive oxygen intermediates increase vascular endothelial growth factor expression in vitro and vivo. J Clin Invest. 1996. 98:1667–1675.
Article30. Hoshi S, Shu Y, Yoshida F, Inagaki T, Sonoda J, Watanabe T, et al. Podocyte injury promotes progressive nephropathy in zucker diabetic fatty rats. Lab Invest. 2002. 82:25–35.
Article31. Foppiano M, Lombardo G. Worldwide pharmacovigilance system and tolrestat withdrawal. Lancet. 1997. 349:399–400.32. Otani A, Takagi H, Suzuma K, Honda Y. Angiotensin II potentiates vascular endothelial growth factor-induced angiogenetic activity in retinal microcapillary endothelial cells. Circ Res. 1998. 82:619–628.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Amelioration of Bleomycin-induced Pulmonary Fibrosis of Rats by an Aldose Reductase Inhibitor, Epalrestat
- Upregulation of Aldose Reductase mRNA by Hyperglycemia in Claf Pulmonary Artery Endothelial Cells
- Vascular Endothelial Growth Factor (VEGF) and Advanced Glycation End Products (AGEs) Overexpression in the Retina and Serum and Lens Opacities of Streptozotocin-induced Diabetic Rats
- Proliferation of Cultured Vascular Smooth Muscle Cells(VSMCs) Obtained from Aortas of Insulin Dependent Diabetic Rats
- Impaired endothelium-dependent relaxation is mediated by reduced production of nitric oxide in the streptozotocin-induced diabetic rats