Yonsei Med J.
2010 Sep;51(5):708-716. 10.3349/ymj.2010.51.5.708.
Pseudomonas aeruginosa Exotoxin A Reduces Chemoresistance of Oral Squamous Carcinoma Cell via Inhibition of Heat Shock Proteins 70 (HSP70)
- Affiliations
-
- 1Department of Oral Anatomy, School of Dentistry, Research Institute for Oral Biotechnology, Pusan National University, Yangsan, Korea. ki91000m@pusan.ac.kr
- 2Department of Anatomy, College of Medicine, Kosin University, Busan, Korea.
- 3Department of Oral and Maxillofacial Surgery, School of Dentistry, Research Institute for Oral Biotechnology, Pusan National University, Yangsan, Korea.
- 4Basic Research Laboratory, Center for Cancer Research, National Cancer Institute, Bethesda, MD, USA.
- KMID: 1071422
- DOI: http://doi.org/10.3349/ymj.2010.51.5.708
Abstract
- PURPOSE
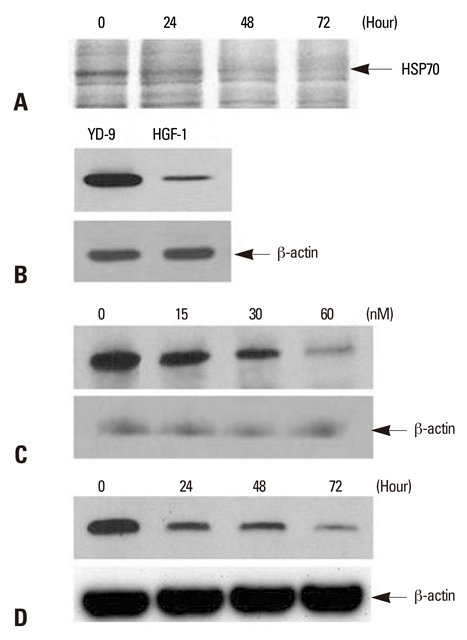
Oral squamous carcinoma (OSCC) cells exhibit resistance to chemotherapeutic agent-mediated apoptosis in the late stage of malignancy. Increased levels of heat shock proteins 70 (HSP70) in cancer cells are known to confer resistance to apoptosis. Since recent advances in the understanding of bacterial toxins have produced new strategies for the treatment of cancers, we investigated the effect of Pseudomonas aeruginosa exotoxin A (PEA) on HSP70 expression and induction of apoptosis in chemoresistant OSCC cell line (YD-9).
MATERIALS AND METHODS
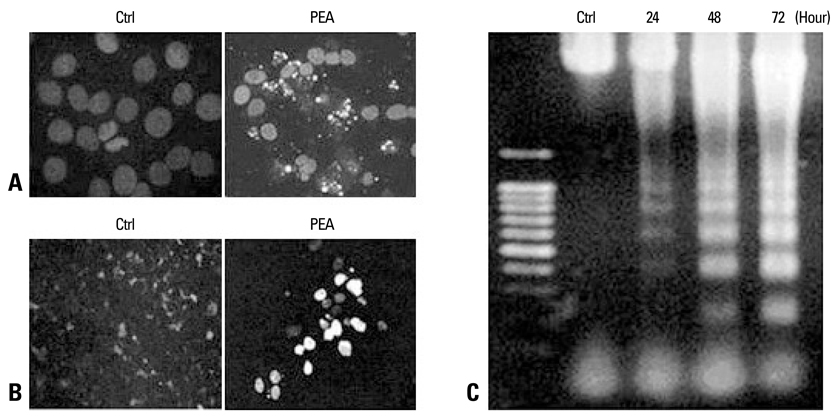
The apoptotic effect of PEA on chemoresistant YD-9 cells was confirmed by MTT, Hoechst and TUNEL stains, DNA electrophoresis, and Western blot analysis.
RESULTS
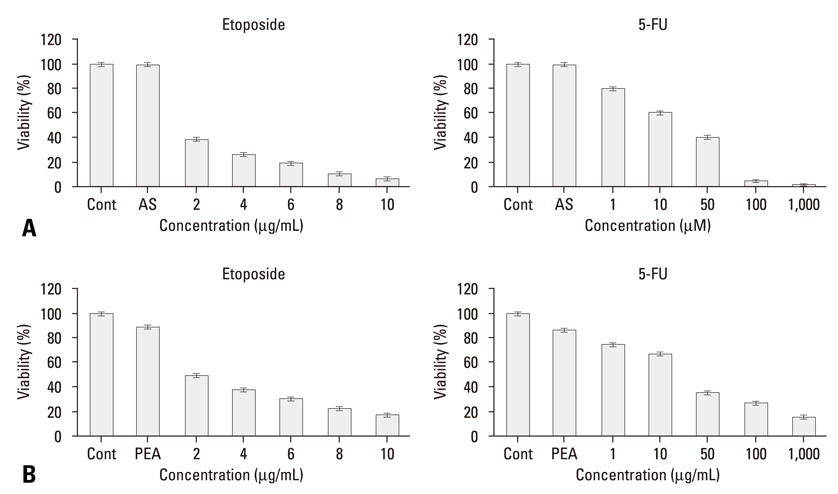
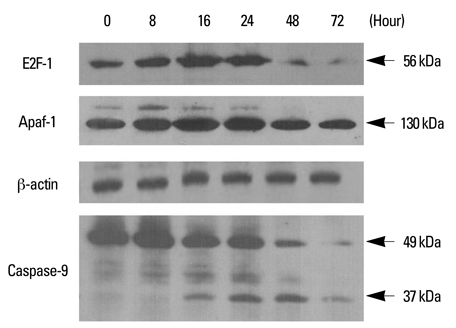
While YD-9 cells showed high resistance to chemotherapeutic agents such as etoposide and 5-fluorouraci (5-FU), HSP70 antisense oligonucelotides sensitized chemoresistant YD-9 cells to etoposide and 5-FU. On the other hand, PEA significantly decreased the viability of YD-9 cells by deteriorating the HSP70-relating protecting system through inhibition of HSP70 expression and inducing apoptosis in YD-9 cells. Apoptotic manifestations were evidenced by changes in nuclear morphology, generation of DNA fragmentation, and activation of caspases. While p53, p21, and E2F-1 were upregulated, cdk2 and cyclin B were downregulated by PEA treatment, suggesting that PEA caused cell cycle arrest at the G2/M checkpoint.
CONCLUSION
Therefore, these results indicate that PEA reduced the chemoresistance through inhibition of HSP70 expression and also induced apoptosis in chemoresistant YD-9 cells.
Keyword
MeSH Terms
-
ADP Ribose Transferases/*pharmacology
Antineoplastic Agents/*pharmacology
Apoptosis/drug effects
Bacterial Toxins/*pharmacology
Blotting, Western
Carcinoma, Squamous Cell/drug therapy/*metabolism
Cell Cycle/drug effects
Cell Line, Tumor
Chromatography, Liquid
Cyclin B/metabolism
Cyclin-Dependent Kinase 2/metabolism
Drug Resistance, Neoplasm/*drug effects
E2F1 Transcription Factor/metabolism
Electrophoresis
Exotoxins/*pharmacology
HSP70 Heat-Shock Proteins/genetics/*metabolism
Humans
In Situ Nick-End Labeling
Mouth Neoplasms/drug therapy/*metabolism
Tandem Mass Spectrometry
Tumor Suppressor Protein p53/metabolism
Virulence Factors/*pharmacology
Figure
Reference
-
1. D'Silva NJ, Ward BB. Tissue biomarkers for diagnosis & management of oral squamous cell carcinoma. Alpha Omegan. 2007. 100:182–189.2. Crowe DL, Sinha UK. p53 apoptotic response to DNA damage dependent on bcl2 but not bax in head and neck squamous cell carcinoma lines. Head Neck. 2006. 28:15–23.
Article3. Tong D, Poot M, Hu D, Oda D. 5-Fluorouracil-induced apoptosis in cultured oral cancer cells. Oral Oncol. 2000. 36:236–241.
Article4. Spierings D, McStay G, Saleh M, Bender C, Chipuk J, Maurer U, et al. Connected to death: the (unexpurgated) mitochondrial pathway of apoptosis. Science. 2005. 310:66–67.
Article5. Beere HM, Green DR. Stress management - heat shock protein-70 and the regulation of apoptosis. Trends Cell Biol. 2001. 11:6–10.
Article6. Rashmi R, Kumar S, Karunagaran D. Human colon cancer cells lacking Bax resist curcumin-induced apoptosis and Bax requirement is dispensable with ectopic expression of Smac or downregulation of Bcl-XL. Carcinogenesis. 2005. 26:713–723.7. Sreedhar AS, Csermely P. Heat shock proteins in the regulation of apoptosis: new strategies in tumor therapy: a comprehensive review. Pharmacol Ther. 2004. 101:227–257.
Article8. Garrido C, Brunet M, Didelot C, Zermati Y, Schmitt E, Kroemer G. Heat shock proteins 27 and 70: anti-apoptotic proteins with tumorigenic properties. Cell Cycle. 2006. 5:2592–2601.
Article9. Li M, Wang J, Jing J, Hua H, Luo T, Xu L, et al. Synergistic promotion of breast cancer cells death by targeting molecular chaperone GRP78 and heat shock protein 70. J Cell Mol Med. 2009. 13:4540–4550.
Article10. Grivicich I, Regner A, Zanoni C, Correa LP, Jotz GP, Henriques JA, et al. Hsp70 response to 5-fluorouracil treatment in human colon cancer cell lines. Int J Colorectal Dis. 2007. 22:1201–1208.
Article11. Bickels J, Kollender Y, Merinsky O, Meller I. Coley's toxin: historical perspective. Isr Med Assoc J. 2002. 4:471–472.12. Zacharski LR, Sukhatme VP. Coley's toxin revisited: immunotherapy or plasminogen activator therapy of cancer? J Thromb Haemost. 2005. 3:424–427.
Article13. Alley SC, Zhang X, Okeley NM, Anderson M, Law CL, Senter PD, et al. The pharmacologic basis for antibody-auristatin conjugate activity. J Pharmacol Exp Ther. 2009. 330:932–938.
Article14. Pastan I. Immunotoxins containing Pseudomonas exotoxin A: a short history. Cancer Immunol Immunother. 2003. 52:338–341.
Article15. Koo HM, VanBrocklin M, McWilliams MJ, Leppla SH, Duesbery NS, Woude GF. Apoptosis and melanogenesis in human melanoma cells induced by anthrax lethal factor inactivation of mitogen-activated protein kinase kinase. Proc Natl Acad Sci U S A. 2002. 99:3052–3057.
Article16. Lee SY, Cherla RP, Caliskan I, Tesh VL. Shiga toxin 1 induces apoptosis in the human myelogenous leukemia cell line THP-1 by a caspase-8-dependent, tumor necrosis factor receptor-independent mechanism. Infect Immun. 2005. 73:5115–5126.
Article17. Cho SJ, Kang NS, Park SY, Kim BO, Rhee DK, Pyo S. Induction of apoptosis and expression of apoptosis related genes in human epithelial carcinoma cells by Helicobacter pylori VacA toxin. Toxicon. 2003. 42:601–611.
Article18. Demir M, Cevahir N, Kaleli I, Yildirim U, Sahin R, Cevik Tepeli E. [Investigation of siderophore, total matrix protease and elastase activity in Pseudomonas aeruginosa isolates from lower respiratory tract and extra-respiratory tract samples.]. Mikrobiyol Bul. 2008. 42:197–208.19. Marra M, Agostinelli E, Tempera G, Lombardi A, Meo G, Budillon A, et al. Anticancer drugs and hyperthermia enhance cytotoxicity induced by polyamine enzymatic oxidation products. Amino Acids. 2007. 33:273–281.
Article20. Kreitman RJ. Immunotoxins for targeted cancer therapy. AAPS J. 2006. 8:E532–E551.
Article21. Andersson Y, Juell S, Fodstad Ø. Downregulation of the antiapoptotic MCL-1 protein and apoptosis in MA-11 breast cancer cells induced by an anti-epidermal growth factor receptor-Pseudomonas exotoxin a immunotoxin. Int J Cancer. 2004. 112:475–483.
Article22. Decker T, Oelsner M, Kreitman RJ, Salvatore G, Wang QC, Pastan I, et al. Induction of caspase-dependent programmed cell death in B-cell chronic lymphocytic leukemia by anti-CD22 immunotoxins. Blood. 2004. 103:2718–2726.
Article23. Lee EJ, Kim J, Lee SA, Kim EJ, Chun YC, Ryu MH, et al. Characterization of newly established oral cancer cell lines derived from six squamous cell carcinoma and two mucoepidermoid carcinoma cells. Exp Mol Med. 2005. 37:379–390.
Article24. Zhang P, Su DM, Liang M, Fu J. Chemopreventive agents induce programmed death-1-ligand 1 (PD-L1) surface expression in breast cancer cells and promote PD-L1-mediated T cell apoptosis. Mol Immunol. 2008. 45:1470–1476.
Article25. Azuma M, Harada K, Supriatno , Tamatani T, Motegi K, Ashida Y, et al. Potentiation of induction of apoptosis by sequential treatment with cisplatin followed by 5-fluorouracil in human oral cancer cells. Int J Oncol. 2004. 24:1449–1455.26. Hsu S, Singh B, Schuster G. Induction of apoptosis in oral cancer cells: agents and mechanisms for potential therapy and prevention. Oral Oncol. 2004. 40:461–473.
Article27. Schümann J, Angermüller S, Bang R, Lohoff M, Tiegs G. Acute hepatotoxicity of Pseudomonas aeruginosa exotoxin A in mice depends on T cells and TNF. J Immunol. 1998. 161:5745–5754.28. Jenkins CE, Swiatoniowski A, Issekutz AC, Lin TJ. Pseudomonas aeruginosa exotoxin A induces human mast cell apoptosis by a caspase-8 and -3-dependent mechanism. J Biol Chem. 2004. 279:37201–37207.
Article29. Li CY, Lee JS, Ko YG, Kim JI, Seo JS. Heat shock protein 70 inhibits apoptosis downstream of cytochrome c release and upstream of caspase-3 activation. J Biol Chem. 2000. 275:25665–25671.30. Gabai VL, Budagova KR, Sherman MY. Increased expression of the major heat shock protein Hsp72 in human prostate carcinoma cells is dispensable for their viability but confers resistance to a variety of anticancer agents. Oncogene. 2005. 24:3328–3338.
Article31. Meyn RE, Milas L, Ang KK. The role of apoptosis in radiation oncology. Int J Radiat Biol. 2009. 85:107–115.
Article32. Moretto P, Hotte SJ. Targeting apoptosis: preclinical and early clinical experience with mapatumumab, an agonist monoclonal antibody targeting TRAIL-R1. Expert Opin Investig Drugs. 2009. 18:311–325.
Article33. Kim SY, Bae YS. Cell death and stress signaling in glycogen storage disease type I. Mol Cells. 2009. 09. 07. [Epub ahead of print].
Article34. Creagh EM, Martin SJ. Caspases: cellular demolition experts. Biochem Soc Trans. 2001. 29:696–702.35. Oancea M, Mazumder S, Crosby ME, Almasan A. Apoptosis assays. Methods Mol Med. 2006. 129:279–290.
Article36. Thant AA, Wu Y, Lee J, Mishra DK, Garcia H, Koeffler HP, et al. Role of caspases in 5-FU and selenium-induced growth inhibition of colorectal cancer cells. Anticancer Res. 2008. 28:3579–3592.37. Wong SH, Santambrogio L, Strominger JL. Caspases and nitric oxide broadly regulate dendritic cell maturation and surface expression of class II MHC proteins. Proc Natl Acad Sci U S A. 2004. 101:17783–17788.
Article38. Harbour JW, Dean DC. The Rb/E2F pathway: expanding roles and emerging paradigms. Genes Dev. 2000. 14:2393–2409.
Article39. Endo-Munoz L, Dahler A, Teakle N, Rickwood D, Hazar-Rethinam M, Abdul-Jabbar I, et al. E2F7 can regulate proliferation, differentiation, and apoptotic responses in human keratinocytes: implications for cutaneous squamous cell carcinoma formation. Cancer Res. 2009. 69:1800–1808.
Article40. Nahle Z, Polakoff J, Davuluri RV, McCurrach ME, Jacobson MD, Narita M, et al. Direct coupling of the cell cycle and cell death machinery by E2F. Nat Cell Biol. 2002. 4:859–864.41. Halaban R, Cheng E, Smicun Y, Germino J. Deregulated E2F transcriptional activity in autonomously growing melanoma cells. J Exp Med. 2000. 191:1005–1016.42. Furukawa Y, Nishimura N, Furukawa Y, Satoh M, Endo H, Iwase S, et al. Apaf-1 is a mediator of E2F-1-induced apoptosis. J Biol Chem. 2002. 277:39760–39768.43. Stevens C, La Thangue NB. The emerging role of E2F-1 in the DNA damage response and checkpoint control. DNA Repair (Amst). 2004. 3:1071–1079.
Article44. Li Y, Sun X, LaMont JT, Pardee AB, Li CJ. Selective killing of cancer cells by beta -lapachone: direct checkpoint activation as a strategy against cancer. Proc Natl Acad Sci U S A. 2003. 100:2674–2678.
Article45. Ismail IA, Kang KS, Lee HA, Kim JW, Sohn YK. Genistein-induced neuronal apoptosis and G2/M cell cycle arrest is associated with MDC1 up-regulation and PLK1 down-regulation. Eur J Pharmacol. 2007. 575:12–20.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Expression of Heat Shock Protein 70 m-RNA in Rat Bladder Overdistended by Diuresis
- The Study about Expression and Regulation Mechanism of Heat Shock Protein 70 by Arisostatins A in Caki Cell Line of Renal Cell Carcinoma
- Heat Shock Protein 70 and Ki-67 Expression in Intraepithelial Neoplasm and Squamous Cell Carcinoma of the Uterine Cervix
- Expression of Heat Shock Protein 70 m-RNA in Non-obstructed Bladder Tissue in Rats with Diuresis
- The Protective Effect of Induced Heat Shock Protein in Human Corneal Epithelial Cells