Yonsei Med J.
2010 Sep;51(5):653-660. 10.3349/ymj.2010.51.5.653.
The Ketogenic Diet Suppresses the Cathepsin E Expression Induced by Kainic Acid in the Rat Brain
- Affiliations
-
- 1Department of Biochemistry, College of Medicine, Kwandong University, Gangneung, Korea. mozart@kd.ac.kr
- 2Department of Anatomy, College of Medicine, Kwandong University, Gangneung, Korea.
- 3Department of Pharmacology, College of Medicine, Kwandong University, Gangneung, Korea.
- KMID: 1071415
- DOI: http://doi.org/10.3349/ymj.2010.51.5.653
Abstract
- PURPOSE
The ketogenic diet has long been used to treat epilepsy, but its mechanism is not yet clearly understood. To explore the potential mechanism, we analyzed the changes in gene expression induced by the ketogenic diet in the rat kainic acid (KA) epilepsy model.
MATERIALS AND METHODS
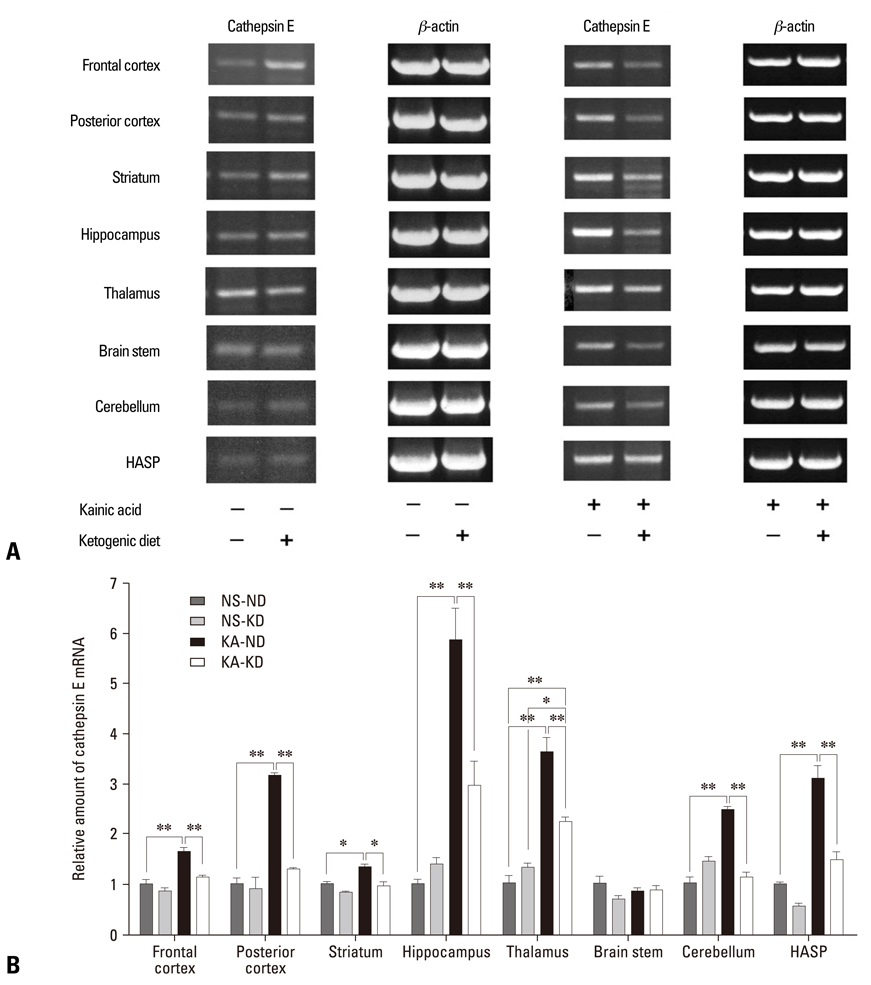
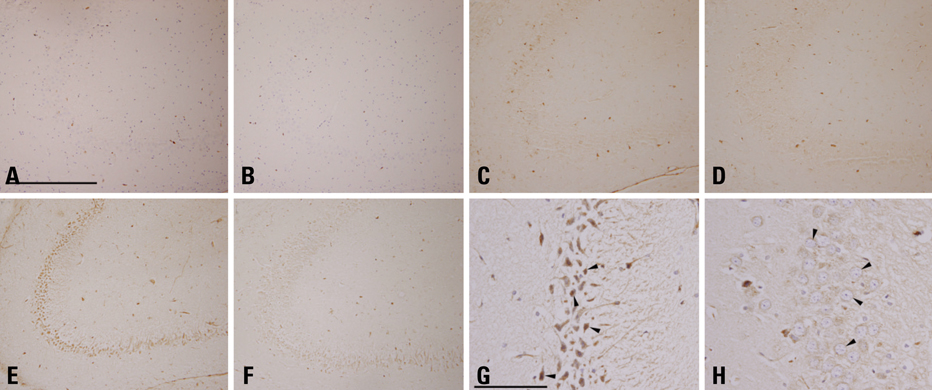
KA-administered rats were fed the ketogenic diet or a normal diet for 4 weeks, and microarray analysis was performed with their brain tissues. The effects of the ketogenic diet on cathepsin E messenger ribonucleic acid (mRNA) expression were analyzed in KA-administered and normal saline-administered groups with semi-quantitative and real-time reverse transcription polymerase chain reaction (RT-PCR). Brain tissues were dissected into 8 regions to compare differential effects of the ketogenic diet on cathepsin E mRNA expression. Immunohistochemistry with an anti-cathepsin E antibody was performed on slides of hippocampus obtained from whole brain paraffin blocks.
RESULTS
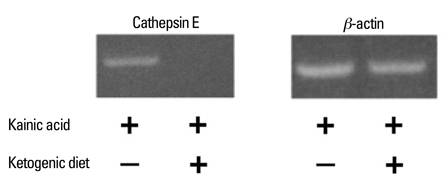
The microarray data and subsequent RT-PCR experiments showed that KA increased the mRNA expression of cathepsin E, known to be related to neuronal cell death, in most brain areas except the brain stem, and these increases of cathepsin E mRNA expression were suppressed by the ketogenic diet. The expression of cathepsin E mRNA in the control group, however, was not significantly affected by the ketogenic diet. The change in cathepsin E mRNA expression was greatest in the hippocampus. The protein level of cathepsin E in the hippocampus of KA-administered rat was elevated in immunohistochemistry and the ketogenic diet suppressed this increase.
CONCLUSION
Our results showed that KA administration increased cathepsin E expression in the rat brain and its increase was suppressed by the ketogenic diet.
Keyword
MeSH Terms
-
3-Hydroxybutyric Acid/blood
Animals
Cathepsin E/genetics/*metabolism
Enzyme Activators/pharmacology
*Gene Expression Regulation, Enzymologic/drug effects
Hippocampus/*drug effects/*metabolism
Immunohistochemistry
Kainic Acid/*pharmacology
*Ketogenic Diet
Male
Oligonucleotide Array Sequence Analysis
Rats
Rats, Sprague-Dawley
Reverse Transcriptase Polymerase Chain Reaction
Figure
Reference
-
1. Wilder RM. The effects of ketonemia on the course of epilepsy. Mayo Clin Bull. 1921. 2:307–308.2. Huffman J, Kossoff EH. State of the ketogenic diet(s) in epilepsy. Curr Neurol Neurosci Rep. 2006. 6:332–340.
Article3. Cross JH, Neal EG. The ketogenic diet-update on recent clinical trials. Epilepsia. 2008. 49:Suppl 8. 6–10.
Article4. Kossoff EH, McGrogan JR. Worldwide use of the ketogenic diet. Epilepsia. 2005. 46:280–289.
Article5. Freeman J, Veggiotti P, Lanzi G, Tagliabue A, Perucca E. Institute Neurology IRCCS C. Mondino Foundation. The ketogenic diet: From molecular mechanisms to clinical effects. Epilepsy Res. 2006. 68:145–180.
Article6. Pisa M, Sanberg PR, Corcoran ME, Fibiger HC. Spontaneously recurrent seizures after intracerebral injections of kainic acid in rat: a possible model of human temporal lobe epilepsy. Brain Res. 1980. 200:481–487.
Article7. Ben-Ari Y. Limbic seizure and brain damage produced by kainic acid: mechanisms and relevance to human temporal lobe epilepsy. Neuroscience. 1985. 14:375–403.
Article8. Schwob JE, Fuller T, Price JL, Olney JW. Widespread patterns of neuronal damage following systemic or intracerebral injection of kainic acid: a histological study. Neuroscience. 1980. 5:991–1014.
Article9. Muller-Schwarze AB, Tandon P, Liu Z, Yang Y, Holmes GL, Stafstrom CE. Ketogenic diet reduces spontaneous seizures and mossy fiber sprouting in the kainic acid model. Neuroreport. 1999. 10:1517–1522.
Article10. Noh HS, Kim YS, Lee HP, Chung KM, Kim DW, Kang SS, et al. The protective effect of a ketogenic diet on kaninic acid-induced hippocampal cell death in the male ICR mice. Epilepsy Res. 2003. 53:119–128.
Article11. Nakanishi H, Tsukuba T, Kondou T, Tanaka T, Yamamoto K. Transient forebrain ischemia induces increased expression and specific localization of cathepsins E and D in rat hippocampus and neostriatum. Exp Neurol. 1993. 121:215–223.
Article12. Nakanishi H, Tominaga K, Amano T, Hirotsu I, Inoue T, Yamamoto K. Age-related changes in activities and localizations of cathepsins D, E, B, and L in the rat brain tissues. Exp Neurol. 1994. 126:119–128.
Article13. Amano T, Nakanishi H, Oka M, Yamamoto K. Increased expression of cathepsins E and D in reactive microglial cells associated with spongiform degeneration in the brain stem of senescence-accelerated mouse. Exp Neurol. 1995. 136:171–182.
Article14. Tominaga K, Nakanishi H, Yasuda Y, Yamamoto K. Excitotoxin-induced neuronal death is associated with response of a unique intracellular aspartic proteinase, cathepsin E. J Neurochem. 1998. 71:2574–2584.
Article15. Bough KJ, Eagles DA. A ketogenic diet increases the resistance to pentylenetetrazole-induced seizures in the rat. Epilepsia. 1999. 40:138–143.
Article16. Su SW, Cilio MR, Sogawa Y, Silveira DC, Homes GL, Stafstrom CE. Timing of ketogenic diet initiation in an experimental epilepsy model. Brain Res Dev Brain Res. 2000. 125:131–138.
Article17. Glowinski J, Iversen LL. Regional studies of catecholamines in the rat brain. I. The disposition of [3H]norepinephrine, [3H]dopamine and [3H] dopa in various regions of the brain. J Neurochem. 1966. 13:655–669.
Article18. Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001. 29:e45.
Article19. Bough KJ, Wetherington J, Hassel B, Pare JF, Gawryluk JW, Greene JG, et al. Mitochondrial biogenesis in the anticonvulsant mechanism of the ketogenic diet. Ann Neurol. 2006. 60:223–235.20. Tai KK, Truong DD. Ketogenic diet prevents seizure and reduces myoclonic jerks in rats with cardiac arrest-induced cerebral hypoxia. Neurosci Lett. 2007. 425:34–38.21. Kim do Y, Rho JM. The ketogenic diet and epilepsy. Curr Opin Clin Nutr Metab Care. 2008. 11:113–120.22. Zaidi N, Hermann C, Herrmann T, Kalbacher H. Emerging functional roles of cathepsin E. Biochem Biophys Res Commun. 2008. 377:327–330.
Article23. Bernstein HG, Wiederanders B. An immunohistochemical study of cathepsin E in Alzheimer-type dementia brains. Brain Res. 1994. 667:287–290.
Article24. Gasior M, Rogawaski MA, Hartman AL. Neuroprotective and disease-modifying effects of the ketogenic diet. Behav Pharmacol. 2006. 17:431–439.
Article25. Halász P, Rásonvi G. Neuroprotection and epilepsy. Adv Exp Med Biol. 2004. 541:91–109.
Article26. Willmore LJ. Antiepileptic drugs and neuroprotection: current status and future roles. Epilepsy Behav. 2005. 7:Suppl 3. S25–S28.27. Fujikawa DG. Prolonged seizures and cellular injury: understanding the connection. Epilepsy Behav. 2005. 7:Suppl 3. S3–S11.
Article28. Meller R, Schindler CK, Chu XP, Xiong ZG, Cameron JA, Simon RP, et al. Seizure-like activity leads to the release of BAD from 14-3-3 protein and cell death in hippocampal neurons in vitro. Cell Death Differ. 2003. 10:539–547.
Article29. Noh HS, Kim YS, Kim YH, Han JY, Park CH, Kang AK, et al. Ketogenic diet protects the hippocampus from kainic acid toxicity by inhibiting the dissociation of bad from 14-3-3. J Neurosci Res. 2006. 84:1829–1836.
Article30. Matarrese P, Straface E, Pietraforte D, Gambardella L, Vona R, Maccaglia A, et al. Peroxynitrite induces senescence and apoptosis of red blood cells through the activation of aspartyl and cysteinyl protease. FASEB J. 2005. 19:416–418.31. Kawakubo T, Okamoto K, Iwata J, Shin M, Okamoto Y, Yasukochi A, et al. Cathespin E prevents tumor growth and metastasis by catalyzing the proteolytic release of soluble TRAIL from tumor cell surface. Cancer Res. 2007. 67:10869–10878.32. Noh HS, Kim DW, Kang SS, Cho GJ, Choi WS. Ketogenic diet prevents clusterin accumulation induced by kainic acid in the hippocampus of male ICR mice. Brain Res. 2005. 1042:114–118.
Article33. Noh HS, Kim DW, Kang SS, Kim YH, Cho GJ, Choi WS. Ketogenic diet decreases the level of proenkephalin mRNA induced by kainic acid in the mouse hippocampus. Neurosci Lett. 2006. 395:87–92.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Light and electron microscopic study on dendrotoxic effect of kainic acid in hippocampus of rat brain
- Effects of kainic Acid-induced seizures on GABA and GABA transporter in the cerebellum of the rat
- Regulation of MAPK Activity by Seizure-induced MKP-1 in Rat Hippocampus
- Comparison between Ketogenic and Diabetic Conventional Diet on Wound Closure in Diabetic Rat Model
- Neuroprotective Effect of N-nitro-L-arginine Methylester Pretreatment on the Early Stage of Kainic Acid Induced Neuronal Degeneration in the Rat Brain