Korean J Ophthalmol.
2011 Aug;25(4):268-274. 10.3341/kjo.2011.25.4.268.
Modulation of Retinal Wound Healing by Systemically Administered Bone Marrow-Derived Mesenchymal Stem Cells
- Affiliations
-
- 1Department of Ophthalmology, Soonchunhyang University Hospital, Soonchunhyang University College of Medicine, Seoul, Korea.
- 2Department of Ophthalmology, Soonchunhyang University Bucheon Hospital, Soonchunhyang University College of Medicine, Bucheon, Korea. tkpark@schmc.ac.kr
- 3Department of Hematology and Oncology, Soonchunhyang University Bucheon Hospital, Soonchunhyang University College of Medicine, Bucheon, Korea.
- KMID: 1018416
- DOI: http://doi.org/10.3341/kjo.2011.25.4.268
Abstract
- PURPOSE
To evaluate whether systemically injected bone marrow-derived mesenchymal stem cells (MSCs) can be incorporated into neuroretinal tissues and play an important role in retinal wound healing in the laser-induced retinal trauma model.
METHODS
Retinotomies were made by applying an Nd:YAG laser to rat retina. On the first day after the injuries, cell suspensions that were obtained from the same line of rat (containing 1 x 10(6) green fluorescence protein [GFP]-marked bone marrow-derived MSCs) were injected through a tail vein in the experimental group and phosphate buffer solution (PBS) was injected in the same way in the control group. Fundus photographs were taken serially for fundus examination and eyeballs were enucleated for histological studies that were conducted at five and seven weeks after MSC and PBS injection. After the tissues were prepared, the retinotomy sites were observed with routine histological staining and confocal microscopy.
RESULTS
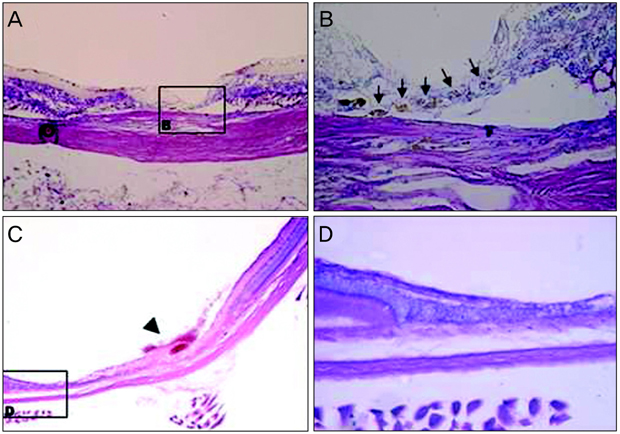
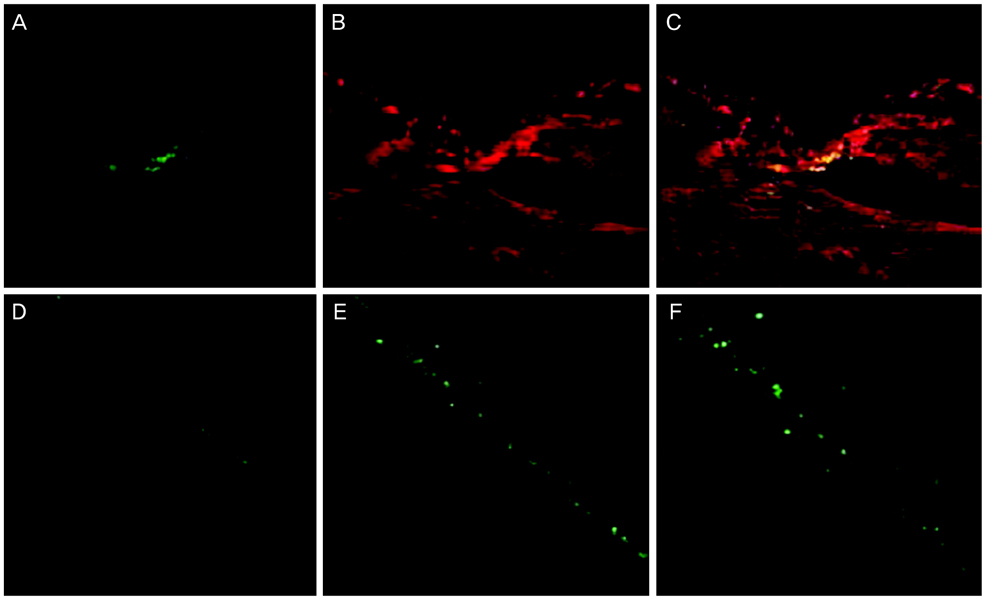
Retinal detachment resolved in the experimental group, whereas it progressed in the control group. The retinotomy sites closed partially with identifiable GFP positive cells 5 weeks after MSC injection. At 7 weeks after MSC injection, complete healing without retinal detachment and plentiful GFP positive cells were observed at the transitional zone between damaged and normal retina.
CONCLUSIONS
Systemically administered GFP-marked MSCs may be incorporated into the neuroretinal tissues and play an important role in the wound modulation of physically damaged retinal tissues.
Keyword
MeSH Terms
Figure
Reference
-
1. Vaananen HK. Mesenchymal stem cells. Ann Med. 2005. 37:469–479.2. Ferrari G, Cusella-De Angelis G, Coletta M, et al. Muscle regeneration by bone marrow-derived myogenic progenitors. Science. 1998. 279:1528–1530.3. Friedenstein AJ, Chailakhjan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970. 3:393–403.4. Krause DS, Theise ND, Collector MI, et al. Multi-organ, multilineage engraftment by a single bone marrow-derived stem cell. Cell. 2001. 105:369–377.5. Lagasse E, Connors H, Al-Dhalimy M, et al. Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nat Med. 2000. 6:1229–1234.6. Orlic D, Kajstura J, Chimenti S, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001. 410:701–705.7. Sanchez-Ramos J, Song S, Cardozo-Pelaez F, et al. Adult bone marrow stromal cells differentiate into neural cells in vitro. Exp Neurol. 2000. 164:247–256.8. Shi Q, Rafii S, Wu MH, et al. Evidence for circulating bone marrow-derived endothelial cells. Blood. 1998. 92:362–367.9. Tomita M, Adachi Y, Yamada H, et al. Bone marrow-derived stem cells can differentiate into retinal cells in injured rat retina. Stem Cells. 2002. 20:279–283.10. Woodbury D, Schwarz EJ, Prockop DJ, Black IB. Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res. 2000. 61:364–370.11. Gartner S, Henkind P. Aging and degeneration of the human macula. 1. Outer nuclear layer and photoreceptors. Br J Ophthalmol. 1981. 65:23–28.12. Fischer AJ, Dierks BD, Reh TA. Exogenous growth factors induce the production of ganglion cells at the retinal margin. Development. 2002. 129:2283–2291.13. Humayun MS, de Juan E Jr, Weiland JD, et al. Pattern electrical stimulation of the human retina. Vision Res. 1999. 39:2569–2576.14. Turner JE, Blair JR. Newborn rat retinal cells transplanted into a retinal lesion site in adult host eyes. Brain Res. 1986. 391:91–104.15. Chacko DM, Rogers JA, Turner JE, Ahmad I. Survival and differentiation of cultured retinal progenitors transplanted in the subretinal space of the rat. Biochem Biophys Res Commun. 2000. 268:842–846.16. Ghosh F, Johansson K, Ehinger B. Long-term full-thickness embryonic rabbit retinal transplants. Invest Ophthalmol Vis Sci. 1999. 40:133–142.17. Nishida A, Takahashi M, Tanihara H, et al. Incorporation and differentiation of hippocampus-derived neural stem cells transplanted in injured adult rat retina. Invest Ophthalmol Vis Sci. 2000. 41:4268–4274.18. Radner W, Sadda SR, Humayun MS, et al. Light-driven retinal ganglion cell responses in blind rd mice after neural retinal transplantation. Invest Ophthalmol Vis Sci. 2001. 42:1057–1065.19. Eglitis MA, Dawson D, Park KW, Mouradian MM. Targeting of marrow-derived astrocytes to the ischemic brain. Neuroreport. 1999. 10:1289–1292.20. Afting M, Stock UA, Nasseri B, et al. Efficient and stable retroviral transfection of ovine endothelial cells with green fluorescent protein for cardiovascular tissue engineering. Tissue Eng. 2003. 9:137–141.21. Wetts R, Fraser SE. Multipotent precursors can give rise to all major cell types of the frog retina. Science. 1988. 239:1142–1145.22. Klassen H, Sakaguchi DS, Young MJ. Stem cells and retinal repair. Prog Retin Eye Res. 2004. 23:149–181.23. Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992. 255:1707–1710.24. Minamino K, Adachi Y, Yamada H, et al. Long-term survival of bone marrow-derived retinal nerve cells in the retina. Neuroreport. 2005. 16:1255–1259.25. Palmer TD, Takahashi J, Gage FH. The adult rat hippocampus contains primordial neural stem cells. Mol Cell Neurosci. 1997. 8:389–404.26. Brancato R, Pratesi R, Leoni G, et al. Histopathology of diode and argon laser lesions in rabbit retina. A comparative study. Invest Ophthalmol Vis Sci. 1989. 30:1504–1510.27. Er H, Doganay S, Turkoz Y, et al. The levels of cytokines and nitric oxide in rabbit vitreous humor after retinal laser photocoagulation. Ophthalmic Surg Lasers. 2000. 31:479–483.28. Gerber HP, Ferrara N. The role of VEGF in normal and neoplastic hematopoiesis. J Mol Med. 2003. 81:20–31.29. Hori Y, Inoue S, Hirano Y, Tabata Y. Effect of culture substrates and fibroblast growth factor addition on the proliferation and differentiation of rat bone marrow stromal cells. Tissue Eng. 2004. 10:995–1005.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Bone Marrow Derived Mesenchymal Stem Cells on Healing of Induced Full-Thickness Skin Wounds in Albino Rat
- Comparison of Human Bone Marrow Stromal Cells with Fibroblasts in Cell Proliferation and Collagen Synthesis
- Role of Angiogenic Stem Cells in Bone Regeneration
- Pharmaceutical Activation of Nrf2 Accelerates Diabetic Wound Healing by Exosomes from Bone Marrow Mesenchymal Stem Cells
- Comparison of Bone Marrow Stromal Cells with Fibroblasts in Wound Healing Accelerating Growth Factor Secretion