Korean J Ophthalmol.
2011 Jun;25(3):189-195. 10.3341/kjo.2011.25.3.189.
Intravenously Administered Anti-recoverin Antibody Alone Does Not Pass through the Blood-Retinal Barrier
- Affiliations
-
- 1Fight against Angiogenesis-Related Blindness (FARB) Laboratory, Department of Ophthalmology, Seoul National University College of Medicine & Seoul Artificial Eye Center, Clinical Research Institute, Seoul National University Hospital, Seoul, Korea. ysyu@s
- 2Department of Radiology, Soonchunhyang University College of Medicine, Bucheon, Korea.
- 3Department of Biochemistry and Molecular Biology, Seoul National University College of Medicine, Seoul, Korea.
- 4NeuroVascular Coordination Research Center, College of Pharmacy and Research Institute of Pharmaceutical Sciences, Seoul National University, Seoul, Korea.
- KMID: 1010027
- DOI: http://doi.org/10.3341/kjo.2011.25.3.189
Abstract
- PURPOSE
Cancer-associated retinopathy is a paraneoplastic retinal degeneration which may primarily result from auto-immune mediated apoptosis. It has been hypothesized that high titer of auto-antibodies are able to cross the blood-retinal barrier (BRB) and to enter retinal cells to activate apoptotic pathway which has been already well-established. However, it still remains to be elucidated whether auto-antibodies could cross BRB in the retina. Herein, we demonstrated that intravenously administrated anti-recoverin antibodies could not pass through BRB and not lead to retinal cell death.
METHODS
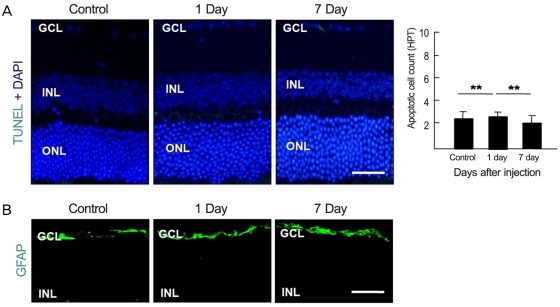
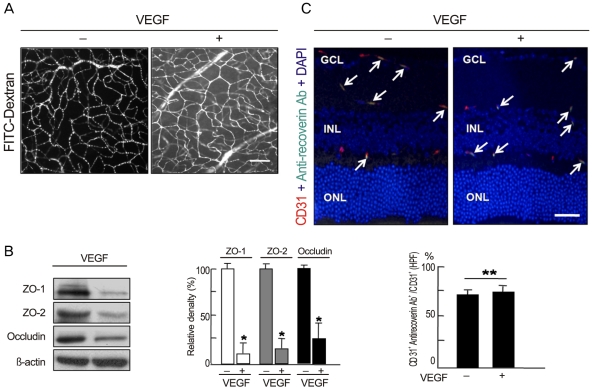
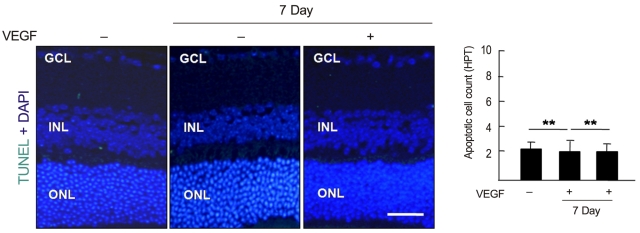
Anti-recoverin antibody was intravenously injected to C57BL/6 mice, which were sacrificed 1 and 7 days to obtain eye. Vascular endothelial growth factor was intravitreally injected to induce BRB breakdown, which was confirmed by fluorescein angiography and western blotting for zonula occludens (ZO)-1, ZO-2 and occludin. To investigate the location of anti-recoverin antibody in the retina, immunofluorescein was performed. The retinal toxicity of intravenous anti-recoverin antibody was evaluated by histological examination and transferase-mediated dUTP nick-end labeling. Immunofluorescein staining for glial fibrillary acidic protein was done to address glial activation as well.
RESULTS
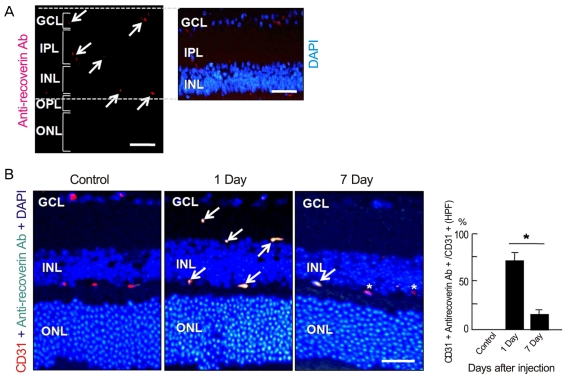
Intravenously administrated anti-recoverin antibodies were exclusively distributed on retinal vessels which were co-localized with CD31, and led to neither increase of glial fibrillary acidic protein expression, as an indicator of retinal stress, nor apoptotic retinal cell death. Moreover, even in the condition of vascular endothelial growth factor-induced BRB breakdown, anti-recoverin antibodies could not migrate across BRB and still remained on retinal vessels without retinal cytotoxicity.
CONCLUSIONS
Our results suggest that high titer of intravascular anti-recoverin antibodies could not penetrate into the retina by themselves, and BRB breakdown mediated by dysregulation of tight junction might not be sufficient to allow anti-recoverin antibodies to pass through BRB.
Keyword
MeSH Terms
Figure
Reference
-
1. Thirkill CE, Roth AM, Keltner JL. Cancer-associated retinopathy. Arch Ophthalmol. 1987; 105:372–375. PMID: 2950846.
Article2. Thirkill CE, FitzGerald P, Sergott RC, et al. Cancer-associated retinopathy (CAR syndrome) with antibodies reacting with retinal, optic-nerve, and cancer cells. N Engl J Med. 1989; 321:1589–1594. PMID: 2555714.
Article3. Khan N, Huang JJ, Foster CS. Cancer associated retinopathy (CAR): an autoimmune-mediated paraneoplastic syndrome. Semin Ophthalmol. 2006; 21:135–141. PMID: 16912011.
Article4. Chen W, Elias RV, Cao W, et al. Anti-recoverin antibodies cause the apoptotic death of mammalian photoreceptor cells in vitro. J Neurosci Res. 1999; 57:706–718. PMID: 10462694.
Article5. Maeda T, Maeda A, Maruyama I, et al. Mechanisms of photoreceptor cell death in cancer-associated retinopathy. Invest Ophthalmol Vis Sci. 2001; 42:705–712. PMID: 11222531.6. Adamus G, Aptsiauri N, Guy J, et al. The occurrence of serum autoantibodies against enolase in cancer-associated retinopathy. Clin Immunol Immunopathol. 1996; 78:120–129. PMID: 8625554.
Article7. Wiechmann AF, Hammarback JA. Expression of recoverin mRNA in the human retina: localization by in situ hybridization. Exp Eye Res. 1993; 57:763–769. PMID: 8150028.
Article8. Bazhin AV, Shifrina ON, Savchenko MS, et al. Low titre autoantibodies against recoverin in sera of patients with small cell lung cancer but without a loss of vision. Lung Cancer. 2001; 34:99–104. PMID: 11557119.
Article9. Bazhin AV, Slepova OS, Tikhomirova NK. Retinal degeneration under the effect of antibodies to recoverin. Bull Exp Biol Med. 2001; 131:350–352. PMID: 11550024.10. Adamus G, Machnicki M, Elerding H, et al. Antibodies to recoverin induce apoptosis of photoreceptor and bipolar cells in vivo. J Autoimmun. 1998; 11:523–533. PMID: 9802939.11. Ohguro H, Ogawa K, Maeda T, et al. Cancer-associated retinopathy induced by both anti-recoverin and anti-hsc70 antibodies in vivo. Invest Ophthalmol Vis Sci. 1999; 40:3160–3167. PMID: 10586938.12. Lee SW, Kim WJ, Choi YK, et al. SeCKS regulates angiogenesis and tight junction formation in blood-brain barrier. Nat Med. 2003; 9:900–906. PMID: 12808449.13. Choi YK, Kim JH, Kim WJ, et al. AKAP12 regulates human blood-retinal barrier formation by downregulation of hypoxia-inducible factor-1alpha. J Neurosci. 2007; 27:4472–4481. PMID: 17442832.14. Kim JH, Kim JH, Park JA, et al. Blood-neural barrier: intercellular communication at glio-vascular interface. J Biochem Mol Biol. 2006; 39:339–345. PMID: 16889675.
Article15. Kim JH, Yu YS, Kim JH, et al. The role of clusterin in in vitro ischemia of human retinal endothelial cells. Curr Eye Res. 2007; 32:693–698. PMID: 17852194.16. Kim JH, Kim JH, Yu YS, et al. Recruitment of pericytes and astrocytes is closely related to the formation of tight junction in developing retinal vessels. J Neurosci Res. 2009; 87:653–659. PMID: 18816791.
Article17. Engelhardt B, Wolburg H. Mini-review: transendothelial migration of leukocytes: through the front door or around the side of the house? Eur J Immunol. 2004; 34:2955–2963. PMID: 15376193.
Article18. Erickson KK, Sundstrom JM, Antonetti DA. Vascular permeability in ocular disease and the role of tight junctions. Angiogenesis. 2007; 10:103–117. PMID: 17340211.
Article19. Lewis GP, Fisher SK. Up-regulation of glial fibrillary acidic protein in response to retinal injury: its potential role in glial remodeling and a comparison to vimentin expression. Int Rev Cytol. 2003; 230:263–290. PMID: 14692684.
Article20. Maeda A, Maeda T, Liang Y, et al. Effects of cytotoxic T lymphocyte antigen 4 (CTLA4) signaling and locally applied steroid on retinal dysfunction by recoverin, cancer-associated retinopathy antigen. Mol Vis. 2006; 12:885–891. PMID: 16917481.21. Riches AC, Sharp JG, Thomas DB, Smith SV. Blood volume determination in the mouse. J Physiol. 1973; 228:279–284. PMID: 4687099.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Outer Blood-retinal Barrier Alteration Induced by Intraocular Ad vanced Glycation Endproduct
- Measurement of Blood Retina Barrier in Branch Retinal Vein Occlusion (BRVO)
- Changes of Blood-Retinal Barrier Induced by Destruction of the Retinal Pigment Epithelium
- The Effect of PRP on the Absorption of Vitreous Hemorrhage in Rabbit Eyes
- Differentiation of Recoverin Immunoreactive Cone Bipolar Cells and Their Timing of Synaptic Formation with GABAergic Amacrine Cells in the Rat Retina