Yonsei Med J.
2013 Jul;54(4):921-926. 10.3349/ymj.2013.54.4.921.
Preoperative Serum Anti-Mullerian Hormone Level in Women with Ovarian Endometrioma and Mature Cystic Teratoma
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Seoul National University Bundang Hospital, Seongnam, Korea. blasto@snubh.org
- 2Department of Obstetrics and Gynecology, Seoul National University College of Medicine, Seoul, Korea.
- 3Institute of Reproductive Medicine and Population, Medical Research Center, Seoul National University, Seoul, Korea.
- 4Department of Obstetrics and Gynecology, Seoul National University Hospital, Seoul, Korea.
- KMID: 2158227
- DOI: http://doi.org/10.3349/ymj.2013.54.4.921
Abstract
- PURPOSE
To investigate whether preoperative serum anti-mullerian hormone (AMH) levels are lower in women with ovarian endometrioma and in women with mature cystic teratoma of the ovaries.
MATERIALS AND METHODS
In a tertiary university hospital, a retrospective case-control study was performed. Serum AMH levels between an advanced (stage III and IV) endometrioma group (n=102) and an age- and body mass index (BMI)-matched control group were compared. Serum AMH levels between an ovarian mature cystic teratoma group (n=48) and age- and BMI-matched controls were also compared.
RESULTS
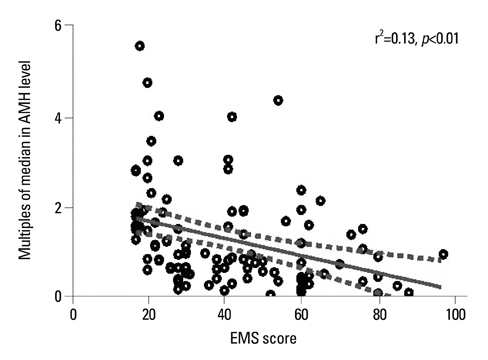
Absolute serum AMH and multiples of the median for AMH (AMH-MoM) relevant to Korean standards were lower in the endometrioma group than controls, but this was not statistically significant (mean+/-SEM, 2.9+/-0.3 ng/mL vs. 3.3+/-0.3 ng/mL, p=0.28 and 1.3+/-0.1 vs. 1.6+/-0.1, p=0.29, respectively). Specifically, the stage IV endometriosis group (n=51) exhibited significantly lower serum AMH and AMH-MoM (2.1+/-0.3 vs. 3.1+/-0.4 ng/mL, p=0.02 and 1.1+/-0.1 vs. 1.7+/-0.2, p=0.03, respectively). Serum AMH and AMH-MoM levels were similar between stage III endometriosis and controls (3.7+/-0.5 vs. 3.4+/-0.5 ng/mL and 1.6+/-0.2 vs. 1.5+/-0.2, respectively), as well as between the mature cystic teratoma group and controls (4.0+/-0.5 ng/mL vs. 4.0+/-0.5 ng/mL and 1.6+/-0.2 vs. 1.6+/-0.3, respectively). Interestingly, AMH-MoM level was negatively correlated with endometriosis score with statistical significance (r2=0.13, p<0.01).
CONCLUSION
In women with advanced ovarian endometrioma, preoperative serum AMH values tended to be lower than those for age and BMI-matched controls. Notably, stage IV endometrioma appeared to be closely associated with decreased ovarian reserve, even before operation. Clinicians should keep this information in mind before undertaking surgery of ovarian endometrioma.
MeSH Terms
Figure
Cited by 1 articles
-
Could surgical management improve the IVF outcomes in infertile women with endometrioma?: a review
Hyun Jong Park, Hannah Kim, Geun Ho Lee, Tae Ki Yoon, Woo Sik Lee
Obstet Gynecol Sci. 2019;62(1):1-10. doi: 10.5468/ogs.2019.62.1.1.
Reference
-
1. Ozkan S, Murk W, Arici A. Endometriosis and infertility: epidemiology and evidence-based treatments. Ann N Y Acad Sci. 2008; 1127:92–100.2. Verkauf BS. Incidence, symptoms, and signs of endometriosis in fertile and infertile women. J Fla Med Assoc. 1987; 74:671–675.3. Navarro J, Garrido N, Remohí J, Pellicer A. How does endometriosis affect infertility? Obstet Gynecol Clin North Am. 2003; 30:181–192.
Article4. Lessey BA. Implantation defects in infertile women with endometriosis. Ann N Y Acad Sci. 2002; 955:265–280.
Article5. Barnhart K, Dunsmoor-Su R, Coutifaris C. Effect of endometriosis on in vitro fertilization. Fertil Steril. 2002; 77:1148–1155.
Article6. Wright VC, Chang J, Jeng G, Macaluso M. Centers for Disease Control and Prevention (CDC). Assisted reproductive technology surveillance--United States, 2005. MMWR Surveill Summ. 2008; 57:1–23.7. Kitajima M, Defrère S, Dolmans MM, Colette S, Squifflet J, Van Langendonckt A, et al. Endometriomas as a possible cause of reduced ovarian reserve in women with endometriosis. Fertil Steril. 2011; 96:685–691.
Article8. Shebl O, Ebner T, Sommergruber M, Sir A, Tews G. Anti muellerian hormone serum levels in women with endometriosis: a case-control study. Gynecol Endocrinol. 2009; 25:713–716.
Article9. Campos CS, Vaamonde D, Andreoli C, Martins AC, Genro VK, Souza CA, et al. Follicular-fluid anti-Müllerian hormone concentration is similar in patients with endometriosis compared with non-endometriotic patients. Reprod Biomed Online. 2010; 21:470–473.
Article10. Chang HJ, Han SH, Lee JR, Jee BC, Lee BI, Suh CS, et al. Impact of laparoscopic cystectomy on ovarian reserve: serial changes of serum anti-Müllerian hormone levels. Fertil Steril. 2010; 94:343–349.
Article11. Iwase A, Hirokawa W, Goto M, Takikawa S, Nagatomo Y, Nakahara T, et al. Serum anti-Müllerian hormone level is a useful marker for evaluating the impact of laparoscopic cystectomy on ovarian reserve. Fertil Steril. 2010; 94:2846–2849.
Article12. Kitajima M, Khan KN, Hiraki K, Inoue T, Fujishita A, Masuzaki H. Changes in serum anti-Müllerian hormone levels may predict damage to residual normal ovarian tissue after laparoscopic surgery for women with ovarian endometrioma. Fertil Steril. 2011; 95:2589–2591.e1.
Article13. Kim MJ, Kim NY, Lee DY, Yoon BK, Choi D. Clinical characteristics of ovarian teratoma: age-focused retrospective analysis of 580 cases. Am J Obstet Gynecol. 2011; 205:32.e1–32.e4.
Article14. Lakkis WG, Martin MC, Gelfand MM. Benign cystic teratoma of the ovary: a 6-year review. Can J Surg. 1985; 28:444–446.15. Parazzini F, La Vecchia C, Negri E, Moroni S, Villa A. Risk factors for benign ovarian teratomas. Br J Cancer. 1995; 71:644–646.
Article16. Lee JY, Jee BC, Lee JR, Kim CH, Park T, Yeon BR, et al. Age-related distributions of anti-Müllerian hormone level and anti-Müllerian hormone models. Acta Obstet Gynecol Scand. 2012; 91:970–975.
Article17. de Vet A, Laven JS, de Jong FH, Themmen AP, Fauser BC. Antimüllerian hormone serum levels: a putative marker for ovarian aging. Fertil Steril. 2002; 77:357–362.
Article18. Kunt C, Ozaksit G, Keskin Kurt R, Cakir Gungor AN, Kanat-Pektas M, Kilic S, et al. Anti-Mullerian hormone is a better marker than inhibin B, follicle stimulating hormone, estradiol or antral follicle count in predicting the outcome of in vitro fertilization. Arch Gynecol Obstet. 2011; 283:1415–1421.
Article19. Muttukrishna S, McGarrigle H, Wakim R, Khadum I, Ranieri DM, Serhal P. Antral follicle count, anti-mullerian hormone and inhibin B: predictors of ovarian response in assisted reproductive technology? BJOG. 2005; 112:1384–1390.
Article20. Bungum L, Jacobsson AK, Rosén F, Becker C, Yding Andersen C, Güner N, et al. Circadian variation in concentration of anti-Müllerian hormone in regularly menstruating females: relation to age, gonadotrophin and sex steroid levels. Hum Reprod. 2011; 26:678–684.
Article21. La Marca A, Stabile G, Artenisio AC, Volpe A. Serum anti-Mullerian hormone throughout the human menstrual cycle. Hum Reprod. 2006; 21:3103–3107.
Article22. Freeman EW, Gracia CR, Sammel MD, Lin H, Lim LC, Strauss JF 3rd. Association of anti-mullerian hormone levels with obesity in late reproductive-age women. Fertil Steril. 2007; 87:101–106.
Article23. Osuga Y, Koga K, Tsutsumi O, Yano T, Maruyama M, Kugu K, et al. Role of laparoscopy in the treatment of endometriosis-associated infertility. Gynecol Obstet Invest. 2002; 53:Suppl 1. 33–39.
Article24. Arici A, Oral E, Bukulmez O, Duleba A, Olive DL, Jones EE. The effect of endometriosis on implantation: results from the Yale University in vitro fertilization and embryo transfer program. Fertil Steril. 1996; 65:603–607.
Article25. Azem F, Lessing JB, Geva E, Shahar A, Lerner-Geva L, Yovel I, et al. Patients with stages III and IV endometriosis have a poorer outcome of in vitro fertilization-embryo transfer than patients with tubal infertility. Fertil Steril. 1999; 72:1107–1109.
Article26. Al-Azemi M, Bernal AL, Steele J, Gramsbergen I, Barlow D, Kennedy S. Ovarian response to repeated controlled stimulation in in-vitro fertilization cycles in patients with ovarian endometriosis. Hum Reprod. 2000; 15:72–75.
Article27. Díaz I, Navarro J, Blasco L, Simón C, Pellicer A, Remohí J. Impact of stage III-IV endometriosis on recipients of sibling oocytes: matched case-control study. Fertil Steril. 2000; 74:31–34.
Article28. Falconer H, Sundqvist J, Gemzell-Danielsson K, von Schoultz B, D'Hooghe TM, Fried G. IVF outcome in women with endometriosis in relation to tumour necrosis factor and anti-Müllerian hormone. Reprod Biomed Online. 2009; 18:582–588.
Article29. Halis G, Arici A. Endometriosis and inflammation in infertility. Ann N Y Acad Sci. 2004; 1034:300–315.
Article30. Lee KS, Joo BS, Na YJ, Yoon MS, Choi OH, Kim WW. Relationships between concentrations of tumor necrosis factor-alpha and nitric oxide in follicular fluid and oocyte quality. J Assist Reprod Genet. 2000; 17:222–228.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Preoperative Serum Anti-Mullerian Hormone Level in Women with Ovarian Endometrioma and Mature Cystic Teratoma
- Serum Anti-Mullerian Hormone Levels before Surgery in Patients with Ovarian Endometriomas Compared to Other Benign Ovarian Cysts
- Clinical Significance of Serum CA 125, CA 19-9 as Tumor Markers in Benign Ovarian Tumors
- Initial Preoperative Hemoglobin Level Affects the Rate of Decline in Anti-Müllerian Hormone Levels after Laparoscopic Ovarian Cystectomy in Women with Ovarian Endometriosis
- Surgical impact on serum anti-Mullerian hormone in women with benign ovarian cyst: A prospective study