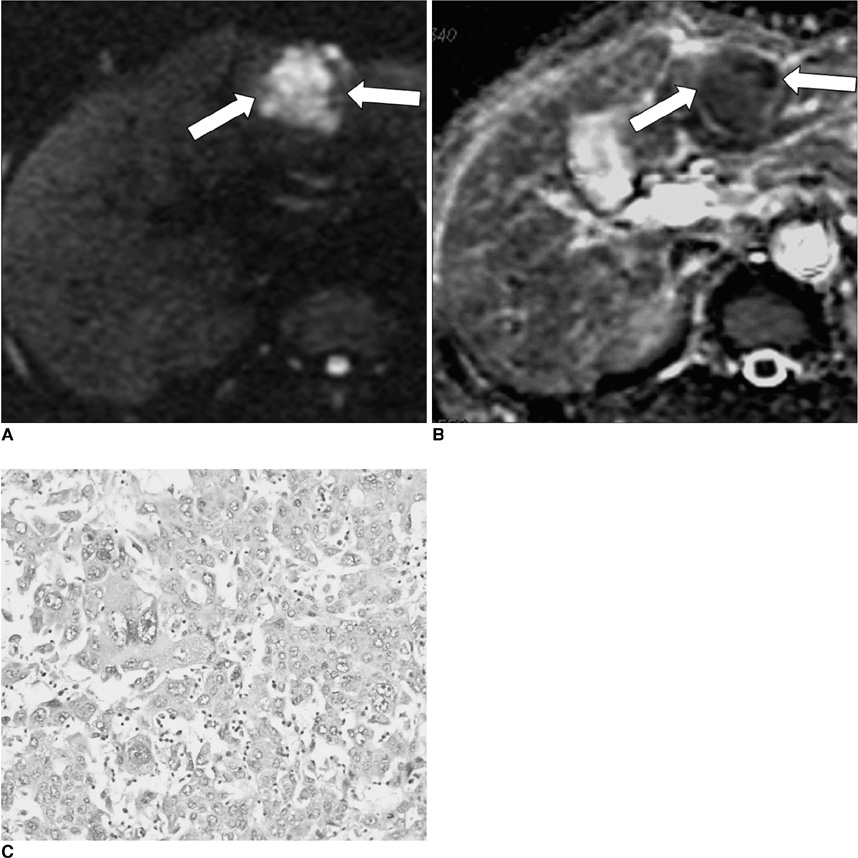
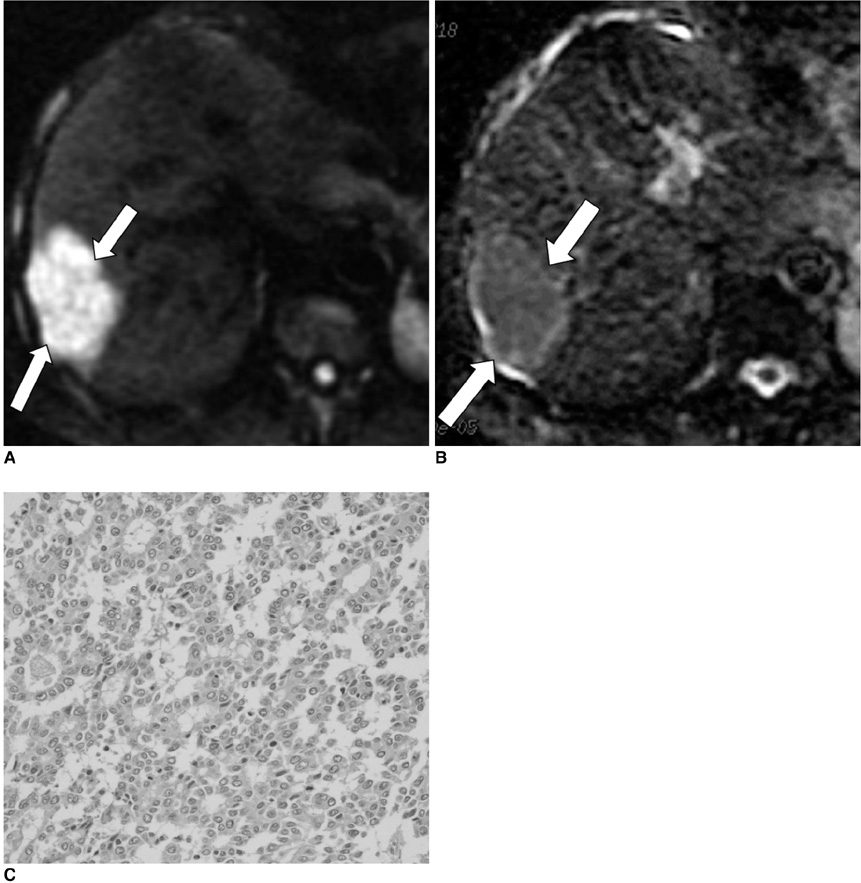
Apparent Diffusion Coefficient Value of Diffusion-Weighted Imaging for Hepatocellular Carcinoma: Correlation with the Histologic Differentiation and the Expression of Vascular Endothelial Growth Factor
- Affiliations
-
- 1Department of Radiology, Chonnam National University Hwasun Hospital, Chonnam National University Medical School, Jeollanam-do 519-763, Korea. yjeong@jnu.ac.kr
- 2Department of Radiology, Chonnam National University Hospital, Chonnam National University Medical School, Gwang-ju 501-746, Korea.
- 3Department of Pathology, Chonnam National University Hwasun Hospital, Chonnam National University Medical School, Jeollanam-do 519-763, Korea.
- 4Department of Surgery, Chonnam National University Hwasun Hospital, Chonnam National University Medical School, Jeollanam-do 519-763, Korea.
- KMID: 946269
- DOI: http://doi.org/10.3348/kjr.2010.11.3.295
Abstract
OBJECTIVE
To evaluate whether the histopathological differentiation and the expression of vascular endothelial growth factor (VEGF) of hepatocellular carcinoma (HCC) do show correlation with the apparent diffusion coefficient (ADC) value on diffusion-weighted imaging (DWI). MATERIALS AND METHODS: Twenty-seven HCCs from 27 patients who had undergone preoperative liver MRI (1.5T) and surgical resection were retrospectively reviewed. DWI was obtained with a single-shot, echo-planar imaging sequence in the axial plane (b values: 0 and 1,000 sec/mm2). On DWIs, the ADC value of the HCCs was measured by one radiologist, who was kept 'blinded' to the histological findings. Histopathologically, the differentiation was classified into well (n = 9), moderate (n = 9) and poor (n = 9). The expression of VEGF was semiquantitatively graded as grade 0 (n = 8), grade 1 (n = 9) and grade 2 (n = 10). We analyzed whether the histopathological differentiation and the expression of VEGF of the HCC showed correlation with the ADC value on DWI. RESULTS: The mean ADC value of the poorly-differentiated HCCs (0.9 +/- 0.13x10(-3) mm2/s) was lower than those of the well-differentiated HCCs (1.2 +/- 0.22x10(-3) mm2/s) (p = 0.031) and moderately-differentiated HCCs (1.1 +/- 0.01x10(-3) mm2/s) (p = 0.013). There was a significant correlation between the differentiation and the ADC value of the HCCs (r = -0.51, p = 0.012). The mean ADC of the HCCs with a VEGF expression grade of 0, 1 and 2 was 1.1 +/- 0.17, 1.1 +/- 0.21 and 1.1 +/- 0.18x10(-3) mm2/s, respectively. The VEGF expression did not show correlation with the ADC value of the HCCs (r = 0.07, p = 0.74). CONCLUSION: The histopathological differentiation of HCC shows inverse correlation with the ADC value. Therefore, DWI with ADC measurement may be a valuable tool for noninvasively predicting the differentiation of HCC.
MeSH Terms
-
Adult
Aged
Carcinoma, Hepatocellular/metabolism/*pathology
Cell Differentiation
Contrast Media/diagnostic use
Diffusion Magnetic Resonance Imaging/*methods
Echo-Planar Imaging/methods
Female
Gadolinium DTPA/diagnostic use
Humans
Image Enhancement/methods
Liver/metabolism/pathology
Liver Neoplasms/metabolism/*pathology
Male
Middle Aged
Predictive Value of Tests
Retrospective Studies
Sensitivity and Specificity
Severity of Illness Index
Vascular Endothelial Growth Factor A/*metabolism
Figure
Cited by 4 articles
-
Pseudoglandular Formation in Hepatocellular Carcinoma Determines Apparent Diffusion Coefficient in Diffusion-Weighted MRI
In Kyung Park, Jeong-Sik Yu, Eun-Suk Cho, Joo Hee Kim, Jae-Joon Chung
Investig Magn Reson Imaging. 2018;22(2):79-85. doi: 10.13104/imri.2018.22.2.79.Pre-Treatment Diffusion-Weighted MR Imaging for Predicting Tumor Recurrence in Uterine Cervical Cancer Treated with Concurrent Chemoradiation: Value of Histogram Analysis of Apparent Diffusion Coefficients
Suk Hee Heo, Sang Soo Shin, Jin Woong Kim, Hyo Soon Lim, Yong Yeon Jeong, Woo Dae Kang, Seok Mo Kim, Heoung Keun Kang
Korean J Radiol. 2013;14(4):616-625. doi: 10.3348/kjr.2013.14.4.616.MRI Features of Hepatocellular Carcinoma Related to Biologic Behavior
Eun-Suk Cho, Jin-Young Choi
Korean J Radiol. 2015;16(3):449-464. doi: 10.3348/kjr.2015.16.3.449.Radiologic features of hepatocellular carcinoma related to prognosis
Shin Hye Hwang, Hyungjin Rhee
J Liver Cancer. 2023;23(1):143-156. doi: 10.17998/jlc.2023.02.16.
Reference
-
1. Ince N, Wands JR. The increasing incidence of hepatocellular carcinoma. N Engl J Med. 1999. 340:798–799.2. Tung-Ping Poon R, Fan ST, Wong J. Risk factors, prevention, and management of postoperative recurrence after resection of hepatocellular carcinoma. Ann Surg. 2000. 232:10–24.3. Haratake J, Takeda S, Kasai T, Nakano S, Tokui N. Predictable factors for estimating prognosis of patients after resection of hepatocellular carcinoma. Cancer. 1993. 72:1178–1183.4. Kim SH, Lee WJ, Lim HK, Park CK. SPIO-enhanced MRI findings of well-differentiated hepatocellular carcinomas: correlation with MDCT findings. Korean J Radiol. 2009. 10:112–120.5. Yamaguchi R, Yano H, Iemura A, Ogasawara S, Haramaki M, Kojiro M. Expression of vascular endothelial growth factor in human hepatocellular carcinoma. Hepatology. 1998. 28:68–77.6. Chedid A, Ryan LM, Dayal Y, Wolf BC, Falkson G. Morphology and other prognostic factors of hepatocellular carcinoma. Arch Pathol Lab Med. 1999. 123:524–528.7. Kadoya M, Matsui O, Takashima T, Nonomura A. Hepatocellular carcinoma: correlation of MR imaging and histopathologic findings. Radiology. 1992. 183:819–825.8. Ebara M, Fukuda H, Kojima Y, Morimoto N, Yoshikawa M, Sugiura N, et al. Small hepatocellular carcinoma: relationship of signal intensity to histopathologic findings and metal content of the tumor and surrounding hepatic parenchyma. Radiology. 1999. 210:81–88.9. Muramatsu Y, Nawano S, Takayasu K, Moriyama N, Yamada N, Yamasaki S, et al. Early hepatocellular carcinoma: MR imaging. Radiology. 1991. 181:209–213.10. Kanematsu M, Osada S, Amaoka N, Goshima S, Kondo H, Kato H, et al. Expression of vascular endothelial growth factor in hepatocellular carcinoma and the surrounding liver and correlation with MRI findings. AJR Am J Roentgenol. 2005. 184:832–841.11. Connolly DT. Vascular permeability factor: a unique regulator of blood vessel function. J Cell Biochem. 1991. 47:219–223.12. Nasu K, Kuroki Y, Tsukamoto T, Nakajima H, Mori K, Minami M. Diffusion-weighted imaging of surgically resected hepatocellular carcinoma: imaging characteristics and relationship among signal intensity, apparent diffusion coefficient, and histopathologic grade. AJR Am J Roentgenol. 2009. 193:438–444.13. Maeda M, Kato H, Sakuma H, Maier SE, Takeda K. Usefulness of the apparent diffusion coefficient in line scan diffusion-weighted imaging for distinguishing between squamous cell carcinomas and malignant lymphomas of the head and neck. AJNR Am J Neuroradiol. 2005. 26:1186–1192.14. Wang J, Takashima S, Takayama F, Kawakami S, Saito A, Matsushita T, et al. Head and neck lesions: characterization with diffusion-weighted echo-planar MR imaging. Radiology. 2001. 220:621–630.15. Sumi M, Sakihama N, Sumi T, Morikawa M, Uetani M, Kabasawa H, et al. Discrimination of metastatic cervical lymph nodes with diffusion-weighted MR imaging in patients with head and neck cancer. AJNR Am J Neuroradiol. 2003. 24:1627–1634.16. Xu H, Li X, Xie JX, Yang ZH, Wang B. Diffusion-weighted magnetic resonance imaging of focal hepatic nodules in an experimental hepatocellular carcinoma rat model. Acad Radiol. 2007. 14:279–286.17. Greene FL, Page DL, Fleming ID, Fritz A, Balch CM, Haller DG, et al. AJCC cancer staging handbook. 2002. 6th ed. New York: Springer.18. Edmondson HA, Steiner PE. Primary carcinoma of the liver: a study of 100 cases among 48,900 necropsies. Cancer. 1954. 7:462–503.19. Kwak BK, Shim HJ, Park ES, Kim SA, Choi D, Lim HK, et al. Hepatocellular carcinoma: correlation between vascular endothelial growth factor level and degree of enhancement by multiphase contrast-enhanced computed tomography. Invest Radiol. 2001. 36:487–492.20. Guo AC, Cummings TJ, Dash RC, Provenzale JM. Lymphomas and high-grade astrocytomas: comparison of water diffusibility and histologic characteristics. Radiology. 2002. 224:177–183.21. Koh DM, Collins DJ. Diffusion-weighted MRI in the body: applications and challenges in oncology. AJR Am J Roentgenol. 2007. 188:1622–1635.22. Roth Y, Tichler T, Kostenich G, Ruiz-Cabello J, Maier SE, Cohen JS, et al. High-b-value diffusion-weighted MR imaging for pretreatment prediction and early monitoring of tumor response to therapy in mice. Radiology. 2004. 232:685–692.23. Park YN, Kim YB, Yang KM, Park C. Increased expression of vascular endothelial growth factor and angiogenesis in the early stage of multistep hepatocarcinogenesis. Arch Pathol Lab Med. 2000. 124:1061–1065.24. Sun HC, Tang ZY. Angiogenesis in hepatocellular carcinoma: the retrospectives and perspectives. J Cancer Res Clin Oncol. 2004. 130:307–319.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Reversal of a Large Ischemic Lesion with Low Apparent Diffusion Coefficient Value by Rapid Spontaneous Recanalization
- Pseudoglandular Formation in Hepatocellular Carcinoma Determines Apparent Diffusion Coefficient in Diffusion-Weighted MRI
- Clinical applications and characteristics of apparent diffusion coefficient maps for the brain of two dogs
- Expression of Vascular Endothelial Growth Factor (VEGF) and Microvessel Density in Hepatocellular Carcinoma
- Diffusion-weighted Imaging and Apparent Diffusion Coefficient Maps for the Evaluation of Pyogenic Ventriculitis