Korean J Ophthalmol.
2005 Dec;19(4):252-257. 10.3341/kjo.2005.19.4.252.
Clinicopathologic Findings after Nasolacrimal Polyurethane Stent Implantations
- Affiliations
-
- 1Department of Ophthalmology, Inje University College of Medicine, Busan, Korea. eyeyang@inje.ac.kr
- 2Department of Pathology, Inje University College of Medicine, Busan, Korea.
- 3Paik Institute for Clinical Research, Inje University, Busan, Korea.
- KMID: 754435
- DOI: http://doi.org/10.3341/kjo.2005.19.4.252
Abstract
- PURPOSE
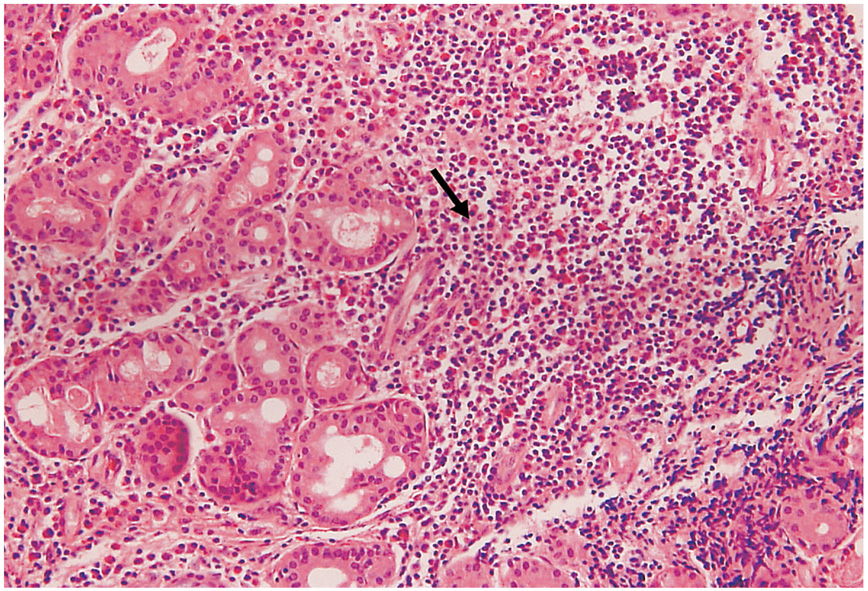
To evaluate the results of nasolacrimal polyurethane stent implantations for the treatment of primary acquired nasolacrimal duct obstruction, and to determine the effects of various surgical procedures, including stent removal, in subsequent nasolacrimal duct obstruction. METHODS: This study included 15 patients who had nasolacrimal polyurethane implantations for the treatment of primary acquired nasolacrimal duct obstruction. Occluded stents were removed either by nasal endoscopy or during dacryocystorhinostomy (DCR). Cultures and biopsies were performed on the removed stents, and the results of the secondary DCR were analyzed for a 6-month follow-up period. RESULTS: During stent removal surgery, various degrees of chronic inflammatory reaction and fibrous tissue formation were detected in the lacrimal sac and nasolacrimal duct. Formations of granuloma and fibrous tissue were found in 15 eyes, and culture-positive reaction were found in nine of the 15 eyes. Conventional dacryocystorhinostomy surgery was performed in nine of the 15 eyes and a silicone tube was located at the canaliculi. Subjective and objective outcome were favorable in 13 of the 15 eyes. CONCLUSIONS: The success rate of nasolacrimal polyurethane stent implantation for the treatment of primary acquired nasolacrimal duct obstruction is low. This may result from a chronic inflammatroy reaction. Despite the low success rate of nasolacrimal polyurethane stent implantation, the success rate of endonasal DCR as a subsequent surgery is favorable.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Results of the Cultured Nasolacrimal Polyurethane Stents (Song's stent®) in Nasolacrimal Duct Obstruction Treatment
Jae Yeong Park, Jong Soo Lee
J Korean Ophthalmol Soc. 2015;56(6):823-829. doi: 10.3341/jkos.2015.56.6.823.Long-term Outcome of Nasolacrimal Duct Obstruction Treated with Nasolacrimal Polyurethane Stents (Song's Stent)
Kyung-Won Suk, Sang-Soo Kim
J Korean Ophthalmol Soc. 2008;49(8):1209-1214. doi: 10.3341/jkos.2008.49.8.1209.
Reference
-
1. Kim BO, Jin KH, Oh JH. Clinical observation on nasolacrimal duct stent intubation on obstruction of nasolacrimal system. J Korean Ophthalmol Soc. 1995. 36:994–999.2. Paul L, Pinto I, Vincente JM. Treatment of complete obstruction of the nasolacrimal system by temporary placement of nasolacrimal polyurethane stents: preliminary results. Clinical Radiology. 2003. 58:876–882.3. Berkefeld J, Kirchner J, Muller HM, et al. Ballon dacryocystoplasty: indications and contraindicstions. Radiology. 1997. 205:785–790.4. Liermann D, Berkefeld J, Fries U, et al. Balloon dacryocystoplasty: an alternative treatment for obstructed tear ducts. Ophthalmologica. 1996. 10:319–324.5. Song HY, Ahn HS, Park ChK, et al. Complete obstruction of the nasolacrimal system. Treatment with Balloon dilatation. Radiology. 1993. 186:367–371.6. Lee JM, Song HY, Han YM, et al. Balloon dacryocystoplasty: results in the treatment of complete and partial obstructions of the nasolacrimal system. Radiology. 1994. 192:503–508.7. Fenton S, Clearly PE, Horan E, et al. Balloon dacryocystoplasty study in the management of adult epiphora. Eye. 2001. 15:67–69.8. Rosen N, Shamir M, Moverman DC, Rosner M. Dacryocystorhinostomy with silicone tubes: evaluation of 253 cases. Ophthalmol Surg. 1989. 20:115–119.9. Yazici Z, Yazici B, Parlak M. Treatment of obstructive epiphora in adults by balloon dacryocystoplasty. Br J Ophthalmol. 1999. 83:692–696.10. Song HY, Jin YH, Kim JH, et al. Nonsurgical placement of a nasolacrimal polyurethane stent. Radiology. 1995. 194:233–237.11. Song HY, Jin YH, Kim JH, et al. Nonsurgical placement of a nasolacrimal polyurethane stent: long-term effectiveness. Radiology. 1996. 200:759–763.12. Pulido-Duque JM, Reyes R, Carreira JM, et al. Treatment of complete and partial obstruction of the nasolacrimal system with polyurethane stents: initial experience. Cardiovasc Intervent Radiol. 1998. 21:41–44.13. Song HY, Lee DH, Ahn H, et al. Lacrimal system obstruction treated with lacrimal polyurethane stents: outcome of removal of occluded stents. Radiology. 1998. 208:689–694.14. Lee JS, Jung G, Oum BS, et al. Clinical efficacy of the polyurethane stent without fluoroscopic guidance in the treatment of nasolacrimal duct obstruction. Ophthalmology. 2000. 107:1666–1670.15. Yazici B, Yazici Z, Parlak M. Treatment of nasolacrimal duct obstruction in adults with polyurethane stent. Am J Ophthalmol. 2001. 131:37–43.16. Pabon IP, Diaz LP, Grande C, de la Cal Lopez MA. Nasolacrimal polyurethane stent placement for epiphora: technical long-term results. J Vasc Interv Radiol. 2001. 12:67–71.17. Perena MF, Castillo J, Medrano J, et al. Nasolacrimal polyurethane stent placement: preliminary results. Eur J Ophthalmol. 2001. 11:25–30.18. Lanciego C, De Miguel S, Perea M, et al. Nasolacrimal stents in the management of epiphora: medium-term results of a multicenter prospective study. J Vasc Interv Radiol. 2001. 12:701–710.19. Paul L, Pinto I, Vicente JM, et al. Nasolacrimal stents in the treatment of epiphora: long-term results. J Vasc Interv Radiol. 2002. 13:83–88.20. Kang SG, Song HY, Lee DH, et al. Nonsurgically placed nasolacrimal stents for epiphora: long-term results and factors favoring stent patency. J Vasc Interv Radiol. 2002. 13:293–300.21. Yazici Z, Yazici B, Parlak M, et al. Treatment of nasolacrimal duct obstruction with polyurethane stent placement: long-term results. Am J Roentgenol. 2002. 179:491–494.22. Munk PL, Lin DT, Morris DC. Epiphora: treatment by means of dacryocystoplasty with balloon dilation of the nasolacrimal drainage apparatus. Radiology. 1990. 177:687–690.23. Janssen AG, Mansour K, Krabbe GJ, et al. Dacryocystoplasty: treatment of epiphora by means of balloon dilation of the obstructed nasolacrimal duct system. Radiology. 1994. 193:453–456.24. Ilgit ET, Yuksel D, Unal M, et al. Transluminal balloon dilatation of the lacrimal drainage system for the treatment of epiphora. Am J Roentgenol. 1995. 165:1517–1524.25. Berkefeld J, Kirchner J, Muller HM, et al. Balloon dacryocystoplasty: indications and contraindications. Radiology. 1997. 205:785–790.26. Lee JM, Song HY, Han YM, et al. Balloon dacryocystoplasty: results in the treatment of complete and partial obstructions of the nasolacrimal system. Radiology. 1994. 192:503–508.27. Yazici Z, Yazici B, Parlak M, et al. Treatment of obstructive epiphora in adults by balloon dacryocystoplasty. Br J Ophthalmol. 1999. 83:692–696.28. Fenton S, Cleary PE, Horan E, et al. Balloon dacryocystoplasty study in the management of adult epiphora. Eye. 2001. 15:67–69.29. Song HY, Ahn HS, Park CK, et al. Complete obstruction of the nasolacrimal system, II: treatment with expandable metallic stents. Radiology. 1993. 186:372–376.30. Ilgit ET, Yuksel D, Unal M, et al. Treatment of recurrent nasolacrimal duct obstructions with balloon-expandable metallic stents: results of early experience. Am J Neuroradiol. 1996. 17:657–663.31. Tucker N, Chow D, Stockl F, et al. Clinically suspected primary acquired nasolacrimal duct obstruction: clinicopathologic review of 150 patients. Ophthalmology. 1997. 104:1882–1886.32. Mauriello JA Jr, Palydowycz S, DeLuca J. Clinicopathologic study of lacrimal sac and nasal mucosa in 44 patients with complete acquired nasolacrimal duct obstruction. Ophthal Plast Reconstr Surg. 1992. 8:13–21.33. Lee-Wing MW, Ashenhurst ME. Clinicopathologic analysis of 166 patients with primary acquired nasolacrimal duct obstruction. Ophthalmology. 2001. 108:2038–2040.34. Bernardini FP, Moin M, Kersten RC, et al. Routine histopathologic evaluation of the lacrimal sac during dacryocystorhinostomy: how useful is it? Ophthalmology. 2002. 109:1214–1218.35. Chaudhry IA, Shamsi FA, Al-Rashed W. Bacteriology of chronic dacryocystitis in a tertiary eye care center. Ophthalmic Plast Reconstr Surg. 2005. 21:207–210.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical Observation on Nasolacrimal Duct Stent Intubation in Obstruction of Nasolacrimal System
- Long-term Outcome of Nasolacrimal Duct Obstruction Treated with Nasolacrimal Polyurethane Stents (Song's Stent)
- Results of the Cultured Nasolacrimal Polyurethane Stents (Song's stent(R)) in Nasolacrimal Duct Obstruction Treatment
- Nasolacrimal Duct Stent Insertion: Causes of Insertion Failure and Reobstruction
- Results with Silicone Stent in Lacrimal Drainage System