Korean J Radiol.
2001 Mar;2(1):21-27. 10.3348/kjr.2001.2.1.21.
Evaluation by Contrast-Enhanced MR Imaging of the Lateral Border Zone in Reperfused Myocardial Infarction in a Cat Model
- Affiliations
-
- 1Univ Ulsan,Coll Med Dept Diagnost Radiol Asan Med Ctr,Songpa Gu,388-1 Poongnap Dong, Seoul 138746, South Korea.
- KMID: 754119
- DOI: http://doi.org/10.3348/kjr.2001.2.1.21
Abstract
OBJECTIVE
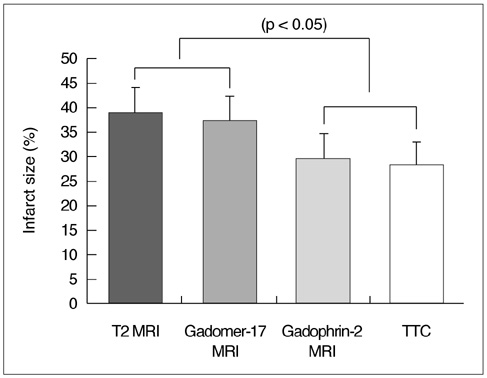
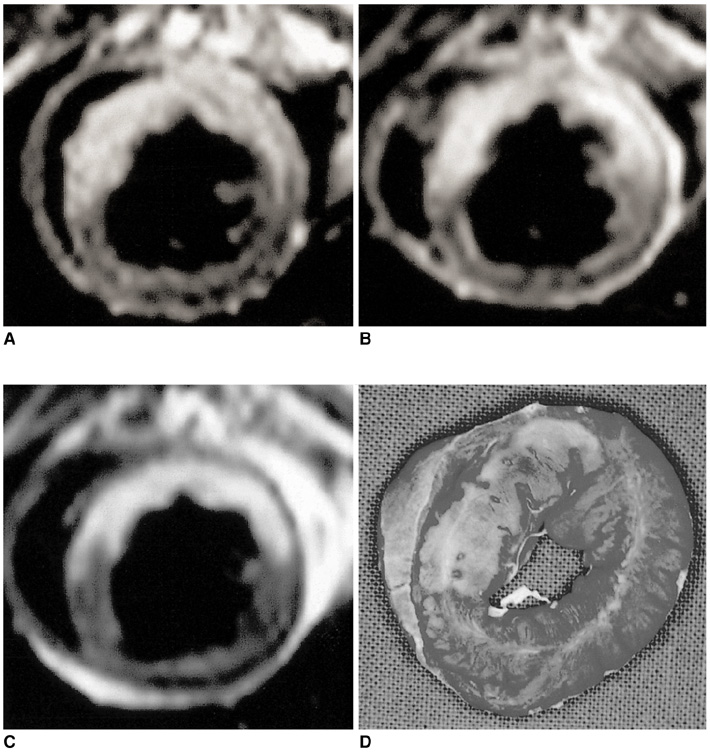
To identify and evaluate the lateral border zone by comparing the size and distribution of the abnormal signal area demonstrated by MR imaging with the infarct area revealed by pathological examination in a reperfused myocardial infarction cat model. MATERIALS AND METHODS: In eight cats, the left anterior descending coronary artery was occluded for 90 minutes, and this was followed by 90 minutes of reper-fusion. ECG-triggered breath-hold turbo spin-echo T2-weighted MR images were initially obtained along the short axis of the heart before the administration of contrast media. After the injection of Gadomer-17 and Gadophrin-2, contrast-enhanced T1-weighted MR images were obtained for three hours. The size of the abnormal signal area seen on each image was compared with that of the infarct area after TTC staining. To assess ultrastructural changes in the myocardium at the infarct area, lateral border zone and normal myocardium, electron microscopic examination was performed. RESULTS: The high signal area seen on T2-weighted images and the enhanced area seen on Gadomer-17-enhanced T1WI were larger than the enhanced area on Gadophrin-2-enhanced T1WI and the infarct area revealed by TTC staining; the difference was expressed as a percentage of the size of the total left ventricle mass (T2= 39.2 %; Gadomer-17 =37.25 % vs Gadophrin-2 = 29.6 %; TTC staining = 28.2 %; p < 0.05). The ultrastructural changes seen at the lateral border zone were compatible with reversible myocardial damage. CONCLUSION: In a reperfused myocardial infarction cat model, the presence and size of the lateral border zone can be determined by means of Gadomer-17- and Gadophrin-2-enhanced MR imaging.
MeSH Terms
Figure
Reference
-
1. Wackers FJ, Bodenheimer M, Fleiss JL, Brown M. Factors affecting uniformity in interpretation of planar thallium-201 imaging in a multicenter trial. The Multicenter Study on Silent Myocardial Ischemia(MSSMI) Thallium-201 Investigator. J Am Coll Cardiol. 1993. 21:1064–1074.2. Klocke FJ. Measurements of coronary blood flow and degree of stenosis : current clinical implications and continuing uncertainties. J Am Coll Cardiol. 1983. 1:31–41.3. Ni Y, Marchal G, Yu J, et al. Localization of metalloporphyrin-induced "specific" enhancement in experimental liver tumors: Comparison of MRI, microangiographic and histologic findings. Acad Radiol. 1995. 2:687–699.4. Hindre F, LePlouzennec M, De Certaines JD, et al. Tetra-pamonophenylporphyrin-conjugated Gd-DTPA: Tumor-specific contrast agent for MR imaging. J Magn Reson Imaging. 1993. 3(1):59–65.5. Young SW, Sidhu MK, Qing F, et al. Preclinical evaluation of gadolinium texaphyrin complex: a new paramagnetic contrast agent for magnetic resonance imaging. Invest Radiol. 1994. 29:330–338.6. Ni Y, Marchal G, Herijgers P, et al. Paramagnetic metalloporphyrins: from enhancers of malignant tumors to markers of myocardial infarcts. Acta Radiol. 1996. 3(2):395–397.7. Noh HN, Choi SI, Choi SH, et al. Gadomer-17 in contrast enhanced MR imaging of reperfused myocardial infarction in a cat model. J Kor Radiol Soc. 2000. 43:539–544.8. Weinmann HJ, Brasch RC, Press WR, Wesby GE. Characteristics of gadolinium-DTPA complex: a potential NMR contrast agent. AJR. 1984. 142:619–624.9. Saeed M, Wendland MF, Yu KK, Li HT, Higgins CB. Dual effects of gadodiamide injection in depiction of the region of myocardial ischemia. J Magn Reson Imaging. 1993. 3:21–29.10. Muller RN, Vander Elst L, Colet JM, et al. Spectroscopic monitoring of the cellular internalization of paramagnetic ions and their complexes: a perfused cells and perfused organs approach. Acad Radiol. 1996. 3:suppl. 2. 277–281.11. Watson AD, Rocklage SM, Carvlin MJ. Stark DD, Bradley WG, editors. Contrast agents. Magnetic resonance imaging. 1992. 2nd ed. St Louis, MO: Mosby;372–437.12. Ni Y, Marchal G, Yu J, et al. Localization of metalloporphyrin-induced specific enhancement in experimental liver tumors: comparison of MRI, microangiographic and histologic findings. Acad Radiol. 1995. 2:687–699.13. Jennings RB, Steenberger C, Reiner KA. Schien FJ, Gimbrone MA, editors. Myocardial ischemia and reperfusion. Cardiovascular pathology. 1995. 1st ed. Baltmore, MD: Williams & Wilkins;58–64.14. Braunwald E. Myocardial reperfusion, limitation of infarct size, reduction of left ventricular dysfunction, and improved survival. Should the paradigm be expanded? Circulation. 1989. 79:441–444.15. Whiteman G, Kieval R, Wetstein L, et al. The relationship between global myocardial redox state and high-energy phosphate profile: a phosphorus-31 nuclear magnetic resonance study. J Sur Res. 1983. 35:339.16. Reimer KA, Lowe JE, Rasmussen MM, Jennings RB. The "wavefront phenomenon" of ischemic cell death. I: Myocardial infarct size vs duration of coronary occlusion in dogs. Circulation. 1977. 56:786–794.17. Reimer KA, Jennings RB. The "wavefront phenomenon" of myocardial ischemic cell death. II: transmural progression of necrosis within the framework of ischemic bed size(myocardium at risk) and collateral flow. Lab Invest. 1979. 40:634–644.18. Maxwell SR, Lip GY. Reperfusion injury: a review of the pathophysiology, clinical manifestations and therapeutic options. Int J Card. 1997. 58:95–117.19. Jennings RB, Reimer KA. Factors involved in salvaging ischemic myocardium: effect of reperfusion of arterial blood. Circulation. 1983. 68:25–36.20. Rivas F, Cobb FR, Bache RJ, Grrenfield JC. Relationship between blood flow to ischemic regions and extent of myocardial infarction. Circ Res. 1976. 38:439–447.21. Jugdutt BI, Hutchins GM, Bulkey BH, Pitt B, Becker LC. Effect of indomethacin on collateral blood flow and infarct size in the conscious dog. Circulation. 1979. 59:734–743.22. Gottlieb GJ, Kudo SH, Alonso DR. Ultrastructural characterization of the border zone surrounding early experimental myocardial infarctions in dogs. Am J Pathol. 1981. 103:292–303.23. Yellon DM, Hearse DJ, Crone R, Granelle J. Characterization of the lateral interface between normal and ischemic tissue in the canine heart during evolving myocardial infarction. Am J Cardiol. 1981. 47:1233–1239.24. Factor SM, Okun EM, Kirk ES. The histological lateral border of acute canine myocardial infarction. Circ Res. 1981. 48:640–649.25. Lim T-H, Hong MK, Lee JS, et al. Novel application of breath-hold turbo spin-echo T2 MRI for detection of acute myocardial infarction. J Magn Reson Imaging. 1997. 7:996–1001.26. Johnston D, Thompston R, Liu P. Magnetic resonance imaging during acute myocardial infarction. Am J Cardiol. 1986. 58:214–219.27. Adzamli IK, Blau M, Pfeffer MA, Bavis MA. Phosphonate-modified Gd-DTPA complexes. III: the detection of myocardial infarction by MRI. Magn Reson Med. 1993. 29:505–511.28. De Roos A, Matheijssen NA, Doornbos J, et al. Myocardial infarct size after reperfusion therapy: assessment with Ga-DTPA-enhanced MR imaging. Radiology. 1990. 176:517–521.29. Saeed M, Wendland MF, Matusi T, Higgins CB. Reperfused myocardial infarction on T1- and susceptibility-enhanced MRI: evidence of loss compartmentalization contrast media. Magn Reson Med. 1994. 31:31–39.30. Judd RM, Lugo OC, Aria M, et al. Physiological basis of myocardial contrast enhancement in fast magnetic resonance imaging of 2-day-old reperfused canine infarct. Circulation. 1995. 92:1902–1910.31. Lim T-H, Lee JH, Lee TK, Mun CW. Comparison of gadolinium polylysine and gadopentetate in contrast-enhanced MR imaging of myocardial ischemia-reperfusion in cats. J Kor Radiol Soc. 1995. 33:59–65.32. Choi SH, Jiang CZ, Lee TK, et al. Myocardial assessment during subacute stage after ischemia-reperfusion: Gd-DTPA-polylysine enhanced MR imaging in cats. J Kor Radiol Soc. 1998. 39:1069–1073.33. Marchal G, Ni Y, Flameng W, et al. Paramagnetic metalloporphyrin: infarct-avid contrast agents for diagnosis of acute myocardial infarction by MRI. Eur Radiology. 1996. 6:2–8.34. Choi SI, Choi SH, Kim ST, et al. Irreversibly damaged myocardium at MR imaging with a necrotic tissue-specific contrast agent in a cat model. Radiology. 2000. 215:863–868.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Significance of Perfusion Defect at Myocardial Perfusion MR Imaging in a Cat Model of Acute Reperfused Myocardial Infarction
- Gadomer-17 in Contrast Enhanced MR Imaging of Reperfused Myocardial Infarction in a Cat Model
- Myocardial Assessment during Subacute Stage after Ischemia-Reperfusion: Gd-DTPA-polylysine Enhanced MR Imagingin Cats
- MR Imaging of Ischemic Heart Disease
- Advanced Cardiac MR Imaging for Myocardial Characterization and Quantification: T1 Mapping