Korean J Radiol.
2002 Dec;3(4):235-239. 10.3348/kjr.2002.3.4.235.
The Significance of Perfusion Defect at Myocardial Perfusion MR Imaging in a Cat Model of Acute Reperfused Myocardial Infarction
- Affiliations
-
- 1Department of Radiology, Asan Medical Center, University of Ulsan College of Medicine, Korea. thlim@amc.seoul.kr
- KMID: 1118790
- DOI: http://doi.org/10.3348/kjr.2002.3.4.235
Abstract
OBJECTIVE
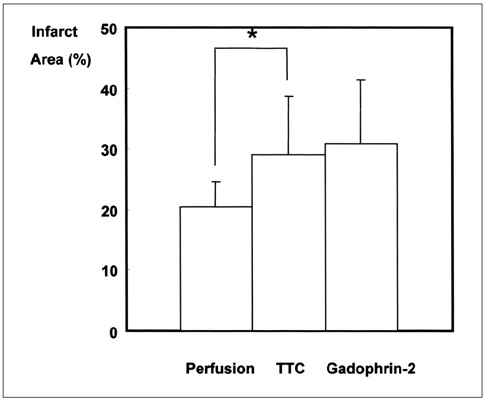
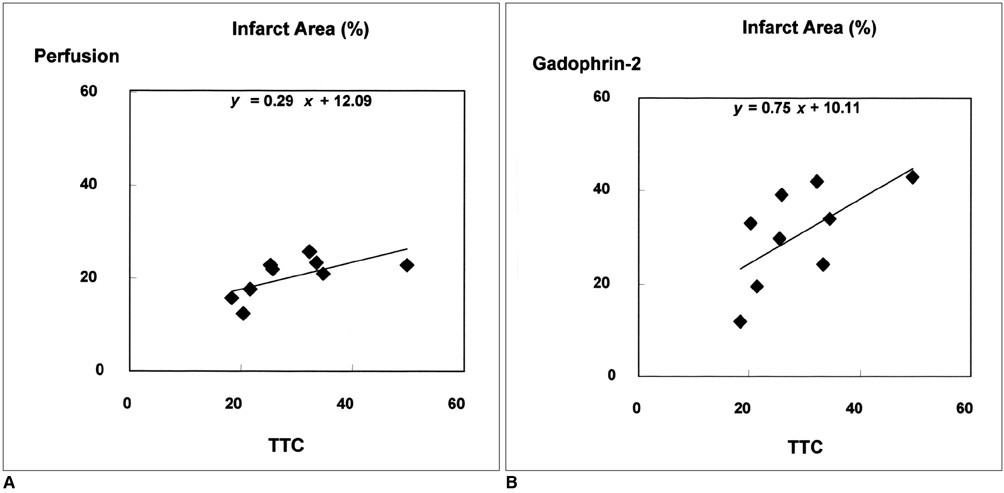
To determine whether the size of a perfusion defect seen at myocardial perfusion MR imaging represents the extent of irreversibly damaged myocardium in acute reperfused myocardial infarction. MATERIALS AND METHODS: In nine cats, reperfused myocardial infarction was induced by occlusion of the left anterior descending coronary artery for 90 minutes and subsequent reperfusion for 90 minutes. At single-slice myocardial perfusion MR imaging at the midventricular level using a turbo-FLASH sequence, 60 short-axis images were sequentially obtained with every heart beat after bolus injection of gadomer-17. The size of the perfusion defect was measured and compared with both the corresponding unstained area seen at triphenyl tetrazolium chloride (TTC) staining and the hyperenhanced area seen at gadophrin-2-enhanced MR imaging performed in the same cat six hours after myocardial perfusion MR imaging. RESULTS: The sizes of perfusion defects seen at gadomer-17-enhanced perfusion MR imaging, unstained areas at TTC staining, and hyperenhanced areas at gadophrin-2-enhanced MR imaging were 20.4+/-4.3%, 29.0+/-9.7%, and 30.7+/-10.6% of the left ventricular myocardium, respectively. The perfusion defects seen at myocardial perfusion MR imaging were significantly smaller than the unstained areas at TTC staining and hyperenhanced areas at gadophrin-2-enhanced MR imaging (p < .01). The sizes of both the perfusion defect at myocardial perfusion MR imaging and the hyperenhanced area at gadophrin-2- enhanced MR imaging correlated well with the sizes of unstained areas at TTC staining (r = .64, p = .062 and r = .70, p = .035, respectively). CONCLUSION: In this cat model, the perfusion defect revealed by myocardial perfusion MR imaging underestimated the true size of acute reperfused myocardial infarction. The defect may represent a more severely damaged area of infarction and probably has prognostic significance.
Keyword
MeSH Terms
Figure
Reference
-
1. Lim TH, Hong MK, Lee JS, et al. Novel application of breath-hold turbo spin-echo T2 MRI for detection of acute myocardial infarction. J Magn Reson Imaging. 1997. 7:996–1001.2. Higgins CB, Herfkens R, Lipton MJ, et al. Nuclear magnetic resonance imaging of acute myocardial infarction in dogs: alterations in magnetic relaxation times. Am J Cardiol. 1983. 52:184–188.3. Choi SI, Jiang CZ, Lim KH, et al. Application of breath-hold T2-weighted, first-pass perfusion and gadolinium-enhanced T1-weighted MR imaging for assessment of myocardial viability in a pig model. J Magn Reson Imaging. 2000. 11:476–480.4. Pislaru SV, Ni Y, Pislaru C, et al. Noninvasive measurements of infarct size after thrombolysis with a necrosis-avid MRI contrast agent. Circulation. 1999. 99:690–696.5. Wilke N, Simm C, Zhang J, et al. Contrast-enhanced first pass myocardial perfusion imaging. Magn Reson Med. 1993. 29:485–497.6. Judd RM, Lugo-Olivieri CH, Arai M, et al. Physiological basis of myocardial contrast enhancement in fast magnetic resonance images of 2-day-old reperfused canine infarcts. Circulation. 1995. 92:1902–1910.7. Saeed M, Wendland MF, Matusi T, Higgins CB. Reperfused myocardial infarctions on T1- and susceptibility-enhanced MRI: evidence for loss of compartmentalization of contrast media. Magn Reson Med. 1994. 31:31–39.8. Lim TH, Lee JG, Lee TK, Mun CW. Comparison of gadolinium polylysine and gadopentetate in contrast-enhanced MR imaging of myocardial ischemia-reperfusion in cats. J Korean Radiol Soc. 1995. 33:59–65.9. Choi SI, Choi SH, Kim ST, et al. Irreversibly damaged myocardium at MR imaging with a necrotic tissue-specific contrast agent in a cat model. Radiology. 2000. 215:863–868.10. Saeed M, Bremerich J, Wendland MF, Wyttenbach R, Weinmann HJ, Higgins CB. Reperfused myocardial infarction as seen with use of necrosis-specific versus standard extracellular MR contrast media in rats. Radiology. 1999. 213:247–257.11. Rogers WJ, Kramer CM, Geskin G, et al. Early contrast-enhanced MRI predicts late functional recovery after reperfused myocardial infarction. Circulation. 1999. 99:744–750.12. Stillman AE, Wilke N, Jerosch-Herold M. Myocardial viability. Radiol Clin North Am. 1999. 37:361–378.13. Lim T-H, Choi SI. MRI of myocardial infarction. J Magn Reson Imaging. 1999. 10:686–693.14. Ambrosio G, Weisman HF, Mannisi JA, Becker LC. Progressive impairment of regional myocardial perfusion after initial restoration of postischemic blood flow. Circulation. 1989. 80:1846–1861.15. Ito H, Maruyama A, Iwakura K, et al. Clinical implications of the 'no-reflow' phenomenon: a predictor of complications and left ventricular remodeling in reperfused anterior wall myocardial infarction. Circulation. 1996. 93:223–228.16. Wu KC, Zerhouni EA, Judd RM, et al. Prognostic significance of microvascular obstruction by magnetic resonance imaging in patients with acute myocardial infarction. Circulation. 1998. 97:765–772.17. Kroft LJM, de Roos A. Blood pool contrast agents for cardiovascular MR imaging. J Magn Reson Imaging. 1999. 10:395–403.18. Ni Y, Marchal G, Yu J, et al. Localization of metalloporphyrin-induced "specific" enhancement in experimental liver tumors: comparison of magnetic resonance imaging, microangiographic, and histologic findings. Acad Radiol. 1995. 2:687–699.19. Marchal G, Ni Y, Herijgers P, et al. Paramagnetic metalloporphyrins: infarct-avid contrast agents for diagnosis of acute myocardial infarction by MRI. Eur Radiol. 1996. 6:2–8.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Stress Testing and Imaging Protocols for Myocardial Perfusion Studies
- Correlation between Reverse Redistribution and Subendocardial Myocardial Infarction Observed in Myocardial Contrast Echocardiography
- Viable myocardium in reperfused acute myocardial infarction: rest and stress first-pass mr imaging
- MR Imaging of Ischemic Heart Disease
- Diagnosis of Coronary Artery Disease Using Myocardial Perfusion SPECT