Korean J Radiol.
2001 Jun;2(2):75-79. 10.3348/kjr.2001.2.2.75.
Evaluation of the Biodurability of Polyurethane-Covered Stent Using a Flow Phantom
- KMID: 754108
- DOI: http://doi.org/10.3348/kjr.2001.2.2.75
Abstract
OBJECTIVE
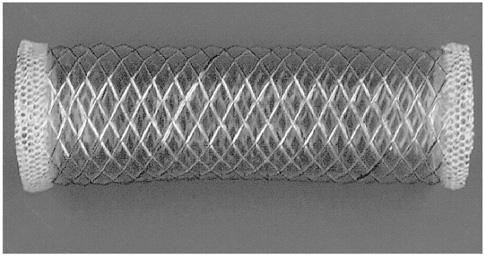
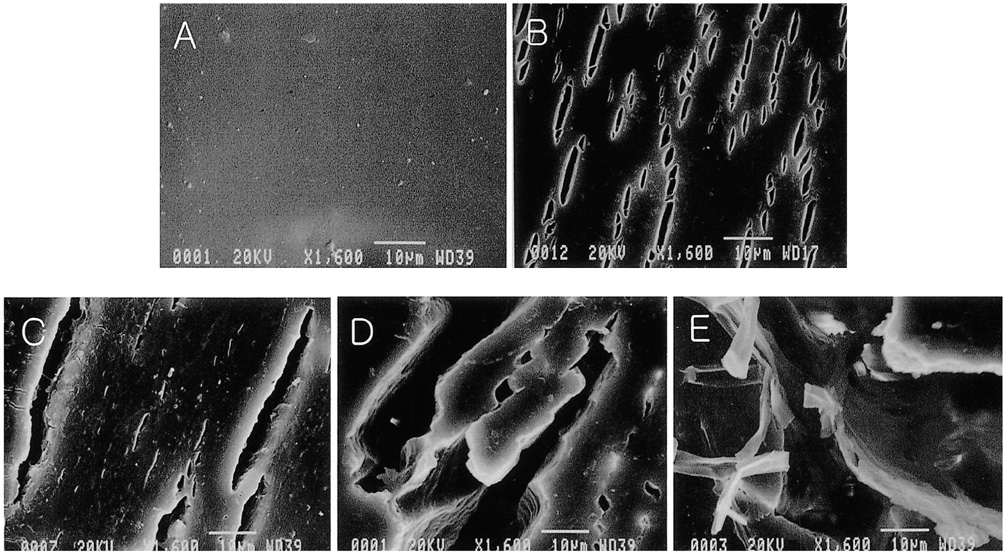
To evaluate the biodurability of the covering material in retrievable metallic stents covered with polycarbonate polyurethane. MATERIALS AND METHODS: Using a peristaltic pump at a constant rate of 1ml/min, bile was recirculated from a reservoir through a long tube containing four stents. Each of these was removed from the system every two weeks and a radial tensile strength test and scanning electron microscopy (SEM) were performed. Each stent, removed at 2, 4, 6 and 8 weeks, was compared with a control stent not exposed to bile juice. RESULTS: Gross examination showed that stents were intact at 2 weeks, but at 4, 6 and 8 weeks cracks were observed. The size of these increased gradually in accordance with the duration of exposure, and at 8 weeks several large holes in the polyurethane membrane were evident. With regard to radial tensile strength, extension and peak load at break were 84.47% and 10.030 N/mm, 54.90% and 6.769 N/mm, 16.55% and 2.452 N/mm, 11.21% and 1.373 N/mm at 0, 2, 4 and 6 weeks, respectively. Scanning electron microscopy at 2 weeks revealed intermittent pitting and cracking, and examination at 4, 6 and 8 weeks showed that the size of these defects was gradually increasing. CONCLUSION: When the polyurethane membrane was exposed to bile, biodegradation was first observed at week two and increased gradually according to the duration of exposure.
Keyword
MeSH Terms
Figure
Reference
-
1. Maccioni F, Rossi M, Salvatori FM, et al. Metallic stents in benign biliary strictures: three -year follow-up. Cardiovasc Intervent Radiol. 1992. 15:360–366.2. Yoon HK, Sung KB, Song HY, et al. Benign biliary strictures associated with recurrent pyogenic cholangitis. AJR. 1997. 169:1523–1527.3. Alvarado R, Palmaz JC, Garcia OJ, Tio FO, Rees CR. Evaluation of polymer-coated balloon-expandable stents in bile ducts. Radiology. 1989. 170:975–978.4. Coons HG. Self-expandible stainless steel biliary stents. Radiology. 1989. 170:979–982.5. Tsang TK, Pollack J, Chodosh HB. Silicone-covered metal stents: an in-vitro evaluation for biofilm formation and patency. Dig Dis Sci. 1999. 44:1780–1785.6. Citron SJ, Martin LG. Benign biliary stricures: treatment with percutaneous cholangioplasty. Radiology. 1991. 178:339–341.7. Vos PM, VanBeek EJ, Smiths NJ, et al. Percutaneous balloon dilatation for benign hepaticojejunostomy strictures. Abdom Imaging. 2000. 25:134–138.8. Gore RM, Levine MS. Textbook of gastrointestinal radiology. 2000. 2nd edition. Philadelphia: WB Saunders Company;1349.9. Rossi P, Salvatori FM, Bezzi M, et al. Percutaneous management of benign biliary strictures with balloon dilation and self-expanding metallic stents. Cardiovasc Intervent Radiol. 1990. 134:231–239.10. Mueller PR, VanSonnenberg E, Ferrucci JT Jr, et al. Biliary stricture dilatation: multicenter review of clinical management in 73 patients. Radiology. 1986. 160:17–22.11. Edwards A, Carson RJ, Szycher M, Bowald S. In-vitro and in-vivo biodurability of a compliant microporous vascular graft. J Biomater Appl. 1998. 13:23–45.12. Severini A, Mantero S, Tanzi MC, et al. In vivo study of polyurethane-coated Gianturco-Rosch biliary Z-stents. Cardiovasc Intervent Radiol. 1999. 22:510–514.13. Mathur AB, Collier TO, Kao WJ, et al. In-vivo biocompatibility and biostability of modified polyurethanes. J Biomed Mater Res. 1997. 36:246–257.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Bile Flow Phantom Model and Animal Bile Duct Dilation Model for Evaluating Biliary Plastic Stents with Advanced Hydrophilic Coating
- Silicone Covered vs. Non-covered Endotracheal Self-expandable Metallic Stent: An Experimental Study
- A New Covered Biliary Metal Stent versus Uncovered Wallstent for Malignant Biliary Obstruction
- Malignant Duodenal Obstructions: Palliative Treatment with Covered Expandable Nitinol Stent
- A Comparison of Covered Expandable Metal Stent and Uncovered Expandable Metal Stent for the Management of Distal Malignant Biliary Obstruction