Korean J Radiol.
2002 Jun;3(2):98-104. 10.3348/kjr.2002.3.2.98.
The Induction of Hyperthermia in Rabbit Liver by means of Duplex Stainless Steel Thermoseeds
- Affiliations
-
- 1Department of Diagnostic Radiology, Institute of Medical Science, Dong-A University College of Medicine, Korea. bhpark@daunet.donga.ac.kr
- 2Medical Radiology Clinic, Korea.
- 3Inje University College of Biomedical Science & Engineering, Korea.
- 4Shine, Ltd.
- KMID: 754068
- DOI: http://doi.org/10.3348/kjr.2002.3.2.98
Abstract
OBJECTIVE
To determine the heating characteristics of needle-shaped duplex stainless steel thermoseeds, and to evaluate their effectiveness in the induction of hyperthermia in rabbit liver.
MATERIALS AND METHODS
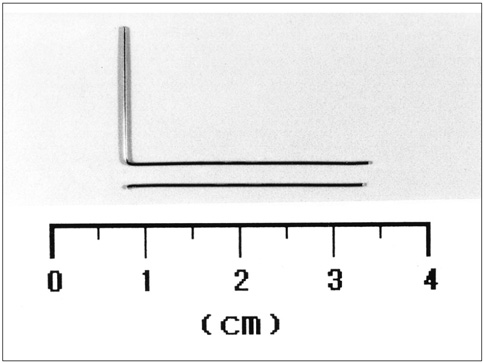
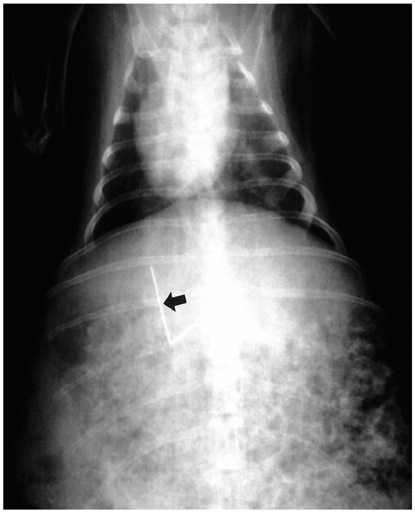
Thermoseeds of the two different shapes, L-shaped for single doses of hyperthermia and I-shaped for in-vitro study and repeated hyperthermic induction, were prepared. For the in-vitro study, an I-shaped thermoseed 0.23 mm in diameter and 25 mm long was placed inside a plastic tube filled with water. Heat was applied for 30 minutes within an induction magnetic field, and during this time changes in temperature were recorded using three thermocouples. For the in-vivo study, fifteen New Zealand white rabbits were divided into five equal groups. An I-shaped or L-shaped thermoseed was inserted in each rabbit's liver, and then placed within the center of the magnetic induction coil during a 30-minute period of hyperthermia. The rabbits in the first group were sacrificed immediately after hyperthermia was induced once, while those in the other groups were sacrificed at 1, 3, and 7 days, respectively, also after one induction. The remaining three rabbits were sacrificed 4 days after three consecutive daily treatment sessions. The resected segments of liver were subsequently evaluated histopathologically for the extent of coagulation necrosis caused by heating of the thermoseed.
RESULTS
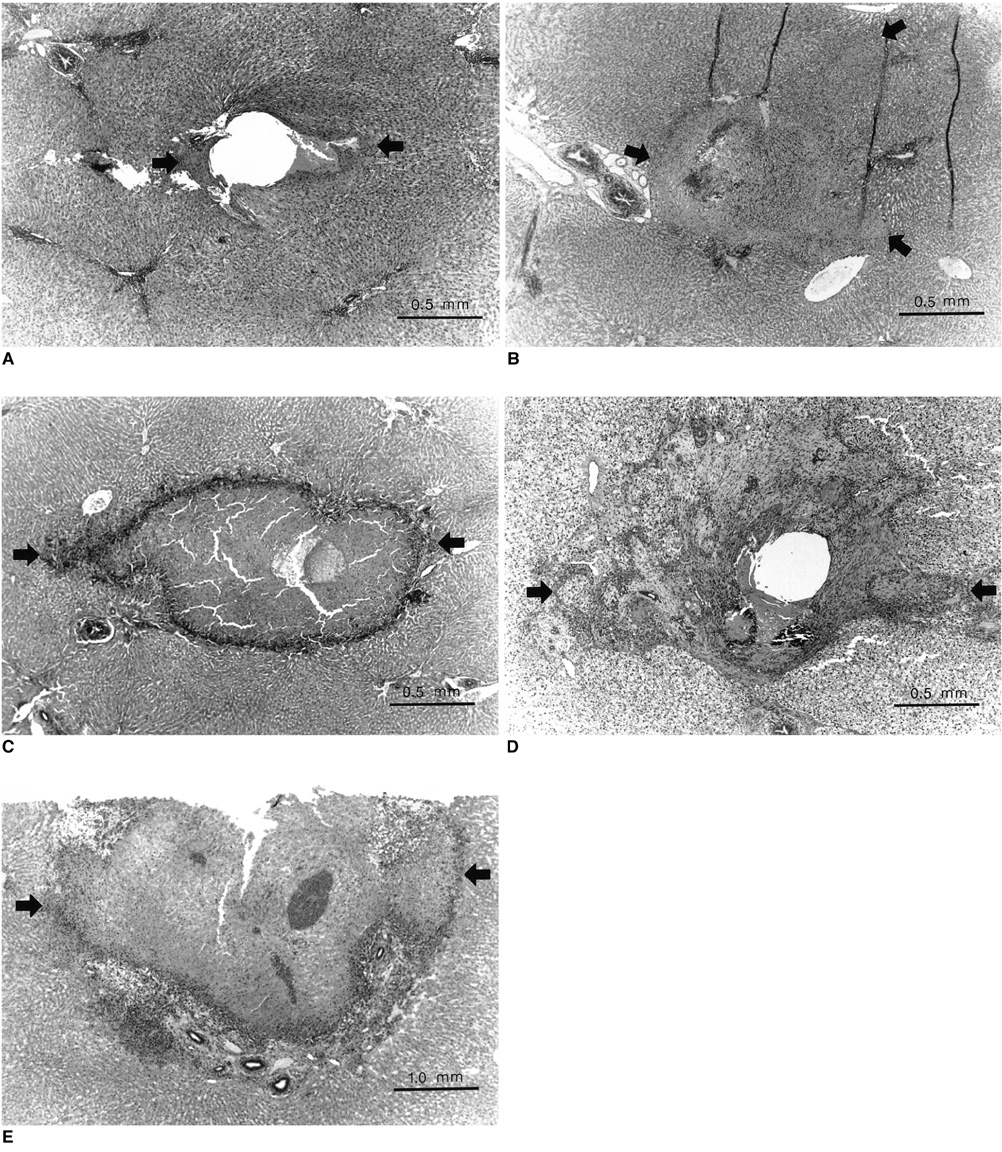
The in-vitro study demonstrated that the temperature in the thermoseed, which was 25.9 degree C before heating and 54.8 degree C after heating, rose rapidly at first but progressively less rapidly as time elapsed. Light microscopic examination of the rabbits' livers revealed coagulation necrosis and infiltration by inflammatory cells around the insertion site of the thermoseed. The maximum diameter of coagulation necrosis was 2.81+/-1.68 mm, and this occurred in the rabbits that were sacrificed 7 days after heat induction.
CONCLUSION
Needle-shaped duplex stainless steel thermoseeds show temperature-dependent-type heating characteristics, and in rabbit liver, induced coagulation necrosis of surrounding tissues after heat is applied for 30 minutes. These thermoseeds may thus be useful for the induction of interstitial hyperthermia.
MeSH Terms
Figure
Reference
-
1. Goldberg SN, Gazelle GS, Mueller PR. Thermal ablation therapy for focal malignancy: a unified approach to underlying principles, techniques, and diagnostic imaging guidance. AJR. 2000. 174:323–331.2. Lim HK. Radiofrequency thermal ablation of hepatocellular carcinomas. Korean J Radiol. 2000. 1:175–184.3. Brezovich IA, Ruby FM. Practical aspect of ferromagnetic thermoseed hyperthermia. Radiol Clin North Am. 1989. 27:589–602.4. Stauffer PR, Cetas TC, Jones RC. Magnetic induction heating of ferromagnetic implant for inducing localized hyperthermia in deep-seated tumors. IEEE Trans Biomed Eng. 1984. 31:76–90.5. Brezovich IA, Atkinson WJ. Temperature distribution in tumor model heated by self-regulating nickel-copper alloy thermoseed. Med Phys. 1984. 11:145–152.6. Matsumoto M, Yoshimura N, Honda Y, Hiraoka M, Ohura K. Ferromagnetic hyperthermia in rabbit eyes using a new glass-ceramic thermoseed. Graefes Arch Clin Exp Ophthalmol. 1994. 232:176–181.7. Molloy JA, Ritter RC, Grady MS, Howard MA, Quate EG, Gillies GT. Experimental determination of the force required for insertion of a thermoseed into deep brain tissue. Ann Biomed Eng. 1990. 18:299–313.8. Takegami K, Sano T, Wakabayashi H, et al. New ferromagnetic bone cement for local hyperthermia. J Biomed Mater Res. 1998. 43:210–214.9. Meredith RF, Brezovich IA, Weppelmann B, et al. Ferromagnetic thermoseed: suitable for an afterloading interstitial implant. Int J Oncol Biol Phys. 1989. 17:1341–1346.10. Seegenschmiedt MH, Brady LW, Sauer R. Interstitial thermoradiotherapy: review of technical and clinical aspect. Am J Clin Oncol. 1990. 13:352–363.11. Larson TR, Bostwick DG, Corica A. Temperature-correlated histopathologic changes following microwave thermoablation of obstructive tissue in patients with benign prostatic hyperplasia. Urology. 1996. 47:463–469.12. Goldberg SN, Hahn PF, Helpern EF, Fogle RM, Gazelle GS. Radio-frequency tissue ablation: effect of pharmacologic modulation of blood flow on coagulation diameter. Radiology. 1998. 209:761–767.13. Wieringen N, Dijk JDP, Snel CE, Cetas TC. Power absorption and temperature control of multi-filament pallidium-nickel thermoseed for interstitial hyperthermia. Phys Med Biol. 1996. 41:2367–2380.14. Wieringen N, Kotte ANT, Leeuwen GMJ, Lagendijk JJW, Dijk JDP, Nieuwenhuys GJ. Dose uniformity of ferromagnetic seed implant in tissue with discrete vasculature: a numerical study of the impact of seed characteristics and implantation techniques. Phys Med Biol. 1998. 43:121–138.15. Tompkins DT, Vanderby R, Klein SA, Beckman WA, Steeves RA, Pailwal BR. Effect of interseed spacing, tissue perfusion, thermoseed temperatures and catheters in ferromagnetic hyperthermia: results from simulations using finite element models of thermoseed and catheters. IEEE Trans Biomed Eng. 1994. 41:975–985.16. Atkinson W, Brezovich IA, Chakraborty DP. Usable frequencies in hyperthermia with thermal seeds. IEEE Trans Biomed Eng. 1984. 31:70–75.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Physical properties and surface topography of orthodontic stainless steel wires: comparing a new Korean product with others from foreign companies
- Stability and Efficacy of Titanium in Osteointegration in a Rabbit Model
- A Stainless Steel Foreign body in the Knee Joint With Synovitis: A Case Report
- Transfemoral Internal Spermatic Vein Occlusion for Varicocele Using a Stainless Steel Spring Coil
- An Experimental Study for The Biomechanical Properties and Histologic Reactions of Carbon Fiber Reinforced Epoxies(CFRE) * Implant