Yonsei Med J.
2008 Jun;49(3):472-478. 10.3349/ymj.2008.49.3.472.
Effect of Itopride Hydrochloride on the Ileal and Colonic Motility in Guinea Pig In Vitro
- Affiliations
-
- 1Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea. HJPARK21@yuhs.ac
- 2Brain Korea 21 Project for Medical Science, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 724264
- DOI: http://doi.org/10.3349/ymj.2008.49.3.472
Abstract
- PURPOSE
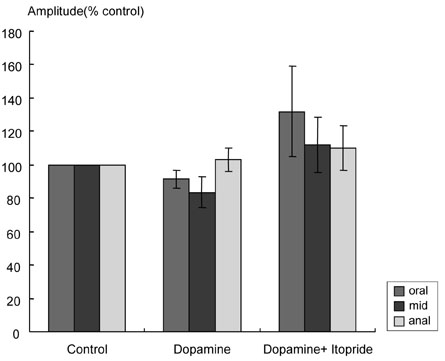
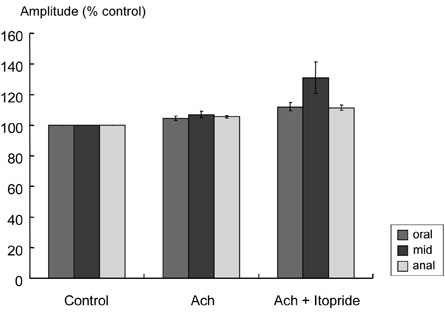
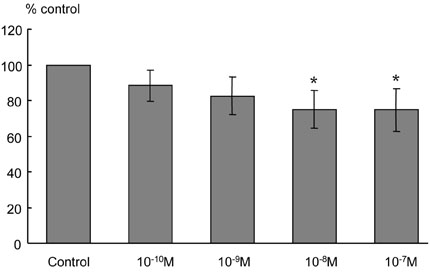
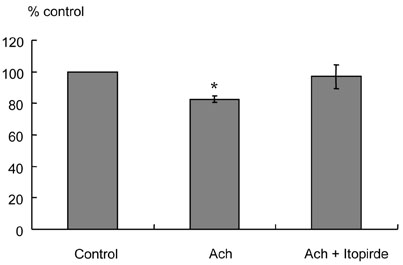
Itopride hydrochloride (itopride) inhibits acetylcholinesterase (AChE) and antagonizes dopamine D(2) receptor, and has been used as a gastroprokinetic agent. However, its prokinetic effect on the small bowel or colon has not yet been thoroughly investigated. The aim of this study was to investigate the effects of itopride on motor functions of the ileum and colon in guinea pigs. MATERIALS AND METHODS: The distal ileum was excised and the activity of peristaltic contraction was determined by measuring the amplitude and propagation velocity of peristaltic contraction. The distal colon was removed and connected to the chamber containing Krebs-Henseleit solution (K-H solution). Artificial fecal matter was inserted into the oral side of the lumen, and moved toward the anal side by intraluminal perfusion via peristaltic pump. Colonic transit times were measured by the time required for the artificial feces to move a total length of 10cm with 2-cm intervals. RESULTS: In the ileum, itopride accelerated peristaltic velocity at higher dosage (10(-10)-10(-6)M) whereas neostigmine accelerated it only with a lower dosage (10(-10)-10(-9)M). Dopamine (10(-8)M) decelerated the velocity that was recovered by itopride infusion. Itopride and neostigmine significantly shortened colonic transit at a higher dosage (10(-10)-10(-6)M). Dopamine (10(-8)M) delayed colonic transit time that was also recovered after infusion of itopride. CONCLUSION: Itopride has prokinetic effects on both the ileum and colon, which are regulated through inhibitory effects on AChE and antagonistic effects on dopamine D(2) receptor.
MeSH Terms
-
Animals
Benzamides/*pharmacology
Benzyl Compounds/*pharmacology
Cholinesterase Inhibitors/pharmacology
Colon/*drug effects/physiology
Dopamine/pharmacology
Dose-Response Relationship, Drug
Gastrointestinal Motility/*drug effects
Guinea Pigs
Ileum/*drug effects/physiology
Neostigmine/pharmacology
Receptors, Dopamine D1/antagonists & inhibitors/physiology
Figure
Reference
-
1. Scratcherd T, Grundy D. The physiology of intestinal motility and secretion. Br J Anaesth. 1984. 56:3–18.
Article2. Li ZS, Schmauss C, Cuenca A, Ratcliffe E, Gershon MD. Physiological modulation of intestinal motility by enteric dopaminergic neurons and the D2 receptor : analysis of dopamine receptor expression, location, development, and function in wild-type and knock-out mice. J Neurosci. 2006. 26:2798–2807.
Article3. Tonini M, Cipollina L, Poluzzi E, Cremas F, Corazza GR, De Ponti F. Review article: clinical implications of enteric and central D2 receptor blockade by antidopaminergic gastrointestinal prokinetics. Aliment Pharmacol Ther. 2004. 19:379–390.
Article4. Tonini M. Recent advances in the pharmacology of gastrointestinal prokinetics. Pharmacol Res. 1996. 33:217–226.
Article5. Schuurkes JA, Van Nueten JM. Domperidone improves myogenically transmitted antroduodenal coordination by blocking dopaminergic receptor sites. Scand J Gastroenterol Suppl. 1984. 96:101–110.6. Sakaguchi J, Nishino H, Ogawa N, Iwanaga Y, Yasuda S, Kato H, et al. Synthesis, gastrointestinal prokinetic activity and structure-activity relationships of novel N-[[2-(dialkylamino) ethoxy]benzyl] benzamide derivatives. Chem Pharm Bull(Tokyo). 1992. 40:202–211.
Article7. Iwanaga Y, Miyashita N, Morikawa K, Mizumoto A, Kondo Y, Itoh Z. A novel water-soluble dopamine-2 antagonist with anticholinesterase activity in gastrointestinal motor activity. Gastroenterology. 1990. 99:401–408.
Article8. Holtmann G, Talley NJ, Liebregts T, Adam B, Parow C. A placebo-controlled trial of itopride in functional dyspepsia. N Engl J Med. 2006. 354:832–840.
Article9. Amarapurkar DN, Rane P. Randomised, double-blind, comparative study to evaluate the efficacy and safety of ganaton (itopride hydrochloride) and mosapride citrate in the management of functional dyspepsia. J Indian Med Assoc. 2004. 102:735–737. 76010. Iwanaga Y, Miyashita N, Saito T, Morikawa K, Itoh Z. Gastroprokinetic effect of a new benzamide derivative itopride and its action mechanisms in conscious dogs. Jpn J Pharmacol. 1996. 71:129–137.
Article11. Tonini M, Galligan JJ, North RA. Effects of cisapride on cholinergic neurotransmission and propulsive motility in the guinea pig ileum. Gastroenterology. 1989. 96(5 Pt 1):1257–1264.12. Ji SW, Park H, Chung JP, Lee SI, Lee YH. Effects of tegaserod on ileal peristalsis of guinea pig in vitro. J Pharmacol Sci. 2004. 94:144–152.
Article13. Ji SW, Park HJ, Cho JS, Lim JH, Lee SI. Investigation into the effects of mosapride on motility of Guinea pig stomach, ileum, and colon. Yonsei Med J. 2003. 44:653–664.14. Iwanaga Y, Kimura T, Miyashita N, Morikawa K, Nagata O, Itoh Z, et al. Characterization of acetylcholinesterase-inhibition by itopride. Jpn J Pharmacol. 1994. 66:317–322.
Article15. Tsubouchi T, Saito T, Mizutani F, Yamauchi T, Iwanaga Y. Stimulatory action of itopride hydrochloride on colonic motor activity in vitro and in vivo. J Pharmacol Exp Ther. 2003. 306:787–793.
Article16. Iwanaga Y, Suzuki N, Kato K, Kimura T, Morikawa K, Kato H, et al. Stimulatory effects of HSR-803 on ileal motor activity. Jpn J Pharmacol. 1993. 62:395–401.
Article17. Mine Y, Yoshikawa T, Oku S, Nagai R, Yoshida H, Hosoki K. Comparison of effect of mosapride citrate and existing 5-HT4 receptor agonists on gastrointestinal motility in vivo and in vitro. J Pharmacol Exp Ther. 1997. 283:1000–1008.18. Fruhwald S, Herk E, Hammer HF, Holzer P, Metzler H. Differential reversal of drug-induced small bowel paralysis by cerulein and neostigmine. Intensive Care Med. 2004. 30:1414–1420.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of Itopride Hydrochloride on the Gastrointestinal Motility in Postoperative Ileus of Guinea Pigs
- Effects of Poncirus fructus on Gastrointestinal Motility in Guinea Pig : in vitro and in vivo Study
- Investigation into the Effects of Mosapride on Motility of Guinea Pig Stomach, Ileum, and Colon
- The Effect of DA-9701 in Opioid-induced Bowel Dysfunction of Guinea Pig
- Exophthalmos Inhibitory Effect of Anti-EPS Serum