Yonsei Med J.
2008 Jun;49(3):429-435. 10.3349/ymj.2008.49.3.429.
Vascular Endothelial Growth Factor Levels in Ascites Between Chemonaive and Chemotreated Patients
- Affiliations
-
- 1Department of Laboratory Medicine, College of Medicine, Catholic University of Korea, Seoul, Korea. chs@catholic.ac.kr
- 2Department of Internal Medicine, College of Medicine, Catholic University of Korea, Seoul, Korea.
- 3Department of Radiation Oncology, College of Medicine, Catholic University of Korea, Seoul, Korea.
- KMID: 724259
- DOI: http://doi.org/10.3349/ymj.2008.49.3.429
Abstract
- PURPOSE
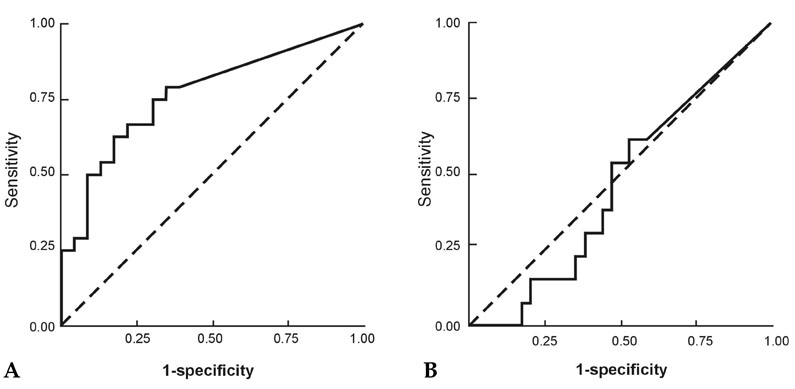
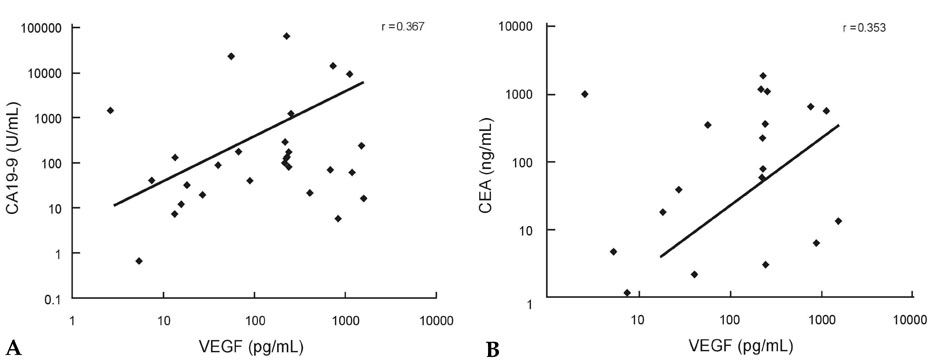
Vascular endothelial growth factor (VEGF) levels in malignant ascites have high diagnostic value for their discrimination from asictes of non-malignant origin. However, there have been no reports on the comparison of VEGF levels between malignant ascites of chemonaive and chemotreated patients. MATERIALS AND METHODS: VEGF levels were measured in 44 ascites patients (cirrhosis ascites, 10; chemonaive patients, 21; chemotreated patients, 13) and compared to the level of carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA 19-9). The diagnostic parameters of sensitivity, specificity, and correlation among 3 markers were evaluated. RESULTS: VEGF levels in malignant ascites of chemonaive and chemotreated patients were significantly higher than those in cirrhotic ascites (p<0.05). VEGF levels in ascites of chemonaive patients were significantly higher than those in chemotreated patients (p<0.05). A cutoff value of 10.4pg/mL was calculated using receiver operating characteristic curves (ROCs) for VEGF in chemotreated and chemonaive patients, which gave sensitivities of 75.0% and 53.8% and specificities of 69.6% and 47.1%, respectively. Positive correlations were observed between VEGF and CEA (r=0.353, p<0.05) as well as between VEGF and CA19-9 (r=0.367, p<0.05) in ascites. CONCLUSION: VEGF levels could be a useful tumor marker for malignant ascites, but its value should carefully be interpreted because of lesser reliability in chemotreated ones.
Keyword
MeSH Terms
Figure
Reference
-
1. Aslam N, Marino CR. Malignant ascites: new concepts in pathophysiology, diagnosis, and management. Arch Intern Med. 2001. 161:2733–2737.2. Colli A, Buccino G, Cocciolo M, Parravicini R, Mariani F, Scaltrini G. Diagnostic accuracy of fibronectin in the differential diagnosis of ascites. Cancer. 1986. 58:2489–2493.
Article3. Sevinc A, Sari R, Fadillioglu E. The utility of lactate dehydrogenase isoenzyme pattern in the diagnostic evaluation of malignant and nonmalignant ascites. J Natl Med Assoc. 2005. 97:79–84.4. Yildirim B, Sari R, Isci N. Patients with spontaneous bacterial peritonitis, and mlignant and cirrhotic ascites. J Natl Med Assoc. 2005. 97:276–280.5. Siddiqui RA, Kochhar R, Singh V, Rajwanshi A, Goenka MK, Metha SK. Evaluation of fibronectin as a marker of malignant ascites. J Gastroenterol Hepatol. 1992. 7:161–164.
Article6. Rana SV, Babu SG, Kocchar R. Usefulness of ascitic fluid cholesterol as a marker for malignant ascites. Med Sci Monit. 2005. 11:CR136–CR142.7. Zebrowski BK, Liu W, Ramirez K, Akagi Y, Mills GB, Ellis LM. Markedly elevated levels of vascular endothelial growth factor in malignant ascites. Ann Surg Oncol. 1999. 6:373–378.
Article8. Dong WG, Sun XM, Yu BP, Luo HS, Yu JP. Role of VEGF and CD44v6 in differentiating benign from malignant ascites. World J Gastroenterol. 2003. 9:2596–2600.
Article9. Abramov Y, Anteby SO, Fasouliotis SJ, Barak V. Markedly elevated levels of vascular endothelial growth factor, fibroblast growth factor, and interleukin 6 in Meigs syndrome. Am J Obstet Gynecol. 2001. 184:354–355.
Article10. Sun XM, Dong WG, Yu BP, Luo HS, Yu JP. Clinical significance of detecting VEGF, CD44v6, MMP-2 and MMP-9 in malignant ascites. Ai Zheng. 2004. 23:85–89.11. Nascimento I, Schaer R, Lemaire D, Freire S, Paule B, Carvalho S, et al. Vascular endothelial growth factor (VEGF) levels as a tool to discriminate between malignant and nonmalignant ascites. APMIS. 2004. 112:585–587.
Article12. Kraft A, Weindel K, Ochs A, Marth C, Zmija J, Schumacher P, et al. Vascular endothelial growth factor in the sera and effusions of patients with malignant and nonmalignant disease. Cancer. 1999. 85:178–187.
Article13. Kuwahara K, Sasaki T, Kobayashi K, Noma B, Serikawa M, Iiboshi T, et al. Gemcitabine suppresses malignant ascites of human pancreatic cancer: correlation with VEGF expression in ascites. Oncol Rep. 2004. 11:73–80.
Article14. Manenti L, Riccardi E, Marchini S, Naumova E, Floriani I, Garofalo A, et al. Circulating plasma vascular endothelial growth factor in mice bearing human ovarian carcinoma xenograft correlates with tumor progression and response to therapy. Mol Cancer Ther. 2005. 4:715–725.
Article15. Keyes K, Cox K, Treadway P, Mann L, Shih C, Faul MM, et al. An in vitro tumor model: analysis of angiogenic factor expression after chemotherapy. Cancer Res. 2002. 62:5597–5602.16. Lissoni P, Fugamalli E, Malugani F, Ardizzoia A, Secondino S, Tancini G, et al. Chemotherapy and angiogenesis in advanced cancer: vascular endothelial growth factor (VEGF) decline as predictor of disease control during taxol therapy in metastatic breast cancer. Int J Biol Markers. 2000. 15:308–311.
Article17. Fernandes LC, Kim SB, Saad SS, Matos D. Value of carcinoembryonic antigen and cytokeratins for the detection of recurrent disease following curative resection of colorectal cancer. World J Gastroenterol. 2006. 12:3891–3894.
Article18. Lowe D, Lee J, Schade R, Chaudhary A. Patient with markedly elevated CA 19-9 not associated with malignancy. South Med J. 2006. 99:306–308.
Article19. Nowak J, Jakubowska D, Wiczkowski A, Sprzaczkowska K, Stechly T, Zmudzińs W, et al. Carbohydrate antigens CA 19-9, CA 242, CA 50 in liver diseases. Wiad Lek. 1998. 51:484–491.20. Trapé J, Molina R, Sant F. Clinical evaluation of the simultaneous determination of tumor markers in fluid and serum and their ratio in the differential diagnosis of serous effusions. Tumour Biol. 2004. 25:276–281.
Article21. Chen SJ, Wang SS, Lu CW, Chao Y, Lee FY, Lee SD, et al. Clinical value of tumour markers and serum-ascites albumin gradient in the diagnosis of malignancy-related ascites. J Gastroenterol Hepatol. 1994. 9:396–400.
Article22. Cascinu S, Del Ferro E, Barbanti I, Ligi M, Fedeli A, Catalano G. Tumor markers in the diagnosis of malignant serous effusions. Am J Clin Oncol. 1997. 20:247–250.
Article23. Miles LE, Hales CM. Labelled antibodies and immunological assay systems. Nature. 1968. 219:186–189.
Article24. Denstman F, Rosen L, Khubchandani IT, Sheets JA, Stasik JJ, Reither RD. Comparing predictive decision rules in postoperative CEA monitoring. Cancer. 1986. 58:2089–2095.
Article25. Del Villano BC, Brennan S, Brock P, Bucher C, Liu V, McClure M, et al. Radioimmunometric assay for a monoclonal antibody-defined tumor marker, CA 19-9. Clin Chem. 1983. 29:549–552.
Article26. Light RW, Macgregor MI, Luchsinger PC, Ball WC Jr. Pleural effusions: the diagnostic separation of transudates and exudates. Ann Intern Med. 1972. 77:507–513.
Article27. WHO Handbook for Reporting Results of Cancer Treatment. 1979. Geneva: World Health Organization;WHO Offset Publication No. 48.28. Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. 1981. 47:207–214.
Article29. Hyodo I, Doi T, Endo H, Hosokawa Y, Nishikawa Y, Tanimizu M, et al. Clinical significance of plasma vascular endothelial growth factor in gastrointestinal cancer. Eur J Cancer. 1998. 34:2041–2045.
Article30. Loewenstein MS, Rittgers RA, Feinerman AE, Kupchik HZ, Marcel BR, Koff RS, et al. Carcinoembryonic antigen assay of ascites and detection of malignancy. Ann Intern Med. 1978. 88:635–638.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Expression of Vascular Endothelial Growth Factor : Clinical Implications in Cervical Neoplasia
- Serum vascular endothelial growth factor as a marker of asthma exacerbation
- Expression of Vascular Endothelial Growth Factor and Peritumoral Brain Edema in Intracranial Meningiomas
- Vascular Endothelial Growth Factor Expression in Human Trohoblast Cell Line
- Relationship between Disease Progression and Angiogenic Cytokine Vascular Endothelial Growth Factor in Patients with Stomach Cancer