Yonsei Med J.
2008 Jun;49(3):405-408. 10.3349/ymj.2008.49.3.405.
Frequency of Pro475Ser Polymorphism of ADAMTS13 Gene and Its Association with ADAMTS-13 Activity in the Korean Population
- Affiliations
-
- 1Department of Internal Medicine, Bundang CHA Hospital, College of Medicine, Pochon CHA University, Sungnam, Korea. doh@cha.ac.kr
- 2Department of Laboratory Medicine, Bundang CHA Hospital, College of Medicine, Pochon CHA University, Sungnam, Korea.
- 3Institute for Clinical Research, Bundang CHA Hospital, College of Medicine, Pochon CHA University, Sungnam, Korea.
- KMID: 724255
- DOI: http://doi.org/10.3349/ymj.2008.49.3.405
Abstract
- PURPOSE
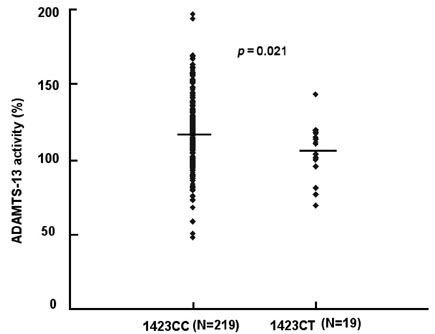
The in vitro study suggested that proline to serine polymorphism in codon 475 (C1423T) of the A Disintegrin and Metalloprotease with ThromboSpondin type 1 repeats-13 (ADAMTS-13) gene is related to reduced activity of ADAMTS- 13. In this study, the frequency of the Pro475Ser polymorphism in Koreans was studied and plasma ADAMTS-13 activity was measured to find out whether this polymorphism contributes to decreased ADAMTS-13 activity in Koreans. PATIENTS AND METHODS: The frequency of the C1423T allele of the ADAMTS13 gene was studied along with measuring plasma ADAMTS-13 activity in 250 healthy Korean individuals. RESULTS: The allele frequency of C1423T polymorphism was 4%, and the median activity of CT type was 107 (69-143)%, which was lower than in controls with the CC genotype [118 (48-197)%, (p=0.021)]. CONCLUSION: Therefore, the Pro475Ser polymorphism seems to be popular in the Korean population, and attenuates ADAMTS-13 plasma activity.
MeSH Terms
Figure
Reference
-
1. Fujikawa K, Suzuki H, McMullen B, Chung D. Purification of human von Willebrand factor-cleaving protease and its identification as a new member of the metalloproteinase family. Blood. 2001. 98:1662–1666.
Article2. Zheng X, Chung D, Takayama TK, Majerus EM, Sadler JE, Fujikawa K. Structure of von Willebrand factor-cleaving protease (ADAMTS13), a metalloprotease involved in thrombotic thrombocytopenic purpura. J Biol Chem. 2001. 276:41059–41063.3. Dong JF, Moake JL, Bernardo A, Fujikawa K, Ball C, Nolasco L, et al. ADAMTS-13 metalloprotease interacts with the endothelial cell-derived ultra-large von Willebrand factor. J Biol Chem. 2003. 278:29633–29639.
Article4. Tsai HM, Lian EC. Antibodies to von Willebrand factor-cleaving protease in acute thrombotic thrombocytopenic purpura. N Engl J Med. 1998. 339:1585–1594.
Article5. Levy GG, Nichols WC, Lian EC, Foroud T, McClintick JN, McGee BM, et al. Mutations in a member of the ADAMTS gene family cause thrombotic thrombocytopenic purpura. Nature. 2001. 413:488–494.
Article6. Tsai HM, Rice L, Sarode R, Chow TW, Moake JL. Antibody inhibitors to von Willebrand factor metalloproteinase and increased binding of von Willebrand factor to platelets in ticlopidine-associated thrombotic thrombocytopenic purpura. Ann Intern Med. 2000. 132:794–799.
Article7. Gerritsen HE, Robles R, Lämmle B, Furlan M. Partial amino acid sequence of purified von Willebrand factor-cleaving protease. Blood. 2001. 98:1654–1661.
Article8. Soejima K, Mimura N, Hirashima M, Maeda H, Hamamoto T, Nakagaki T, et al. A novel human metalloprotease synthesized in the liver and secreted into the blood: possibly, the von Willebrand factor-cleaving protease? J Biochem. 2001. 130:475–480.
Article9. Ginsburg D. Identifying novel genetic determinants of hemostatic balance. J Thromb Haemost. 2005. 3:1561–1568.
Article10. Kokame K, Matsumoto M, Soejima K, Yagi H, Ishizashi H, Funato M, et al. Mutations and common polymorphisms in ADAMTS-13 gene responsible for von Willebrand factor-cleaving protease activity. Proc Natl Acad Sci U S A. 2002. 99:11902–11907.
Article11. Uchida T, Wada H, Mizutani M, Iwashita M, Ishihara H, Shibano T, et al. Identification of novel mutations in ADAMTS13 in an adult patient with congenital thrombotic thrombocytopenic purpura. Blood. 2004. 104:2081–2083.
Article12. Veyradier A, Lavergne JM, Ribba AS, Obert B, Loirat C, Meyer D, et al. Ten candidate ADAMTS13 mutations in six French families with congenital thrombotic thrombocytopenic purpura (Upshaw-Schulman syndrome). J Thromb Haemost. 2004. 2:424–429.
Article13. Ruan C, Dai L, Su J, Wang Z, Ruan C. The frequency of P475S polymorphism in von Willebrand factor-cleaving protease in the Chinese population and its relevance to arterial thrombotic disorders. Thromb Haemost. 2004. 91:1257–1258.
Article14. Bongers TN, de Maat MP, Dippel DW, Uitterlinden AG, Leebeek FW. Absence of Pro475Ser polymorphism in ADAMTS-13 in Caucasians. J Thromb Haemost. 2005. 3:805.
Article15. Kokame K, Nobe Y, Kokubo Y, Okayama A, Miyata T. FRETS-VWF73, a first fluorogenic substrate for ADAMTS13 assay. Br J Haematol. 2005. 129:93–100.
Article16. Bianchi V, Robles R, Alberio L, Furlan M, Lämmle B. Von Willebrand factor-cleaving protease (ADAMTS13) in thrombocytopenic disorders: a severely deficient activity is specific for thrombotic thrombocytopenic purpura. Blood. 2002. 100:710–713.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Thrombotic thrombocytopenic purpura with decreased level of ADAMTS-13 activity and increased level of ADAMTS-13 inhibitor in an adolescent
- ADAMTS13 Gene Mutations in Children with Hemolytic Uremic Syndrome
- Changes in VWF-cleaving Metalloprotease (ADAMTS 13) activity in the thrombotic microangiopathy after kidney tranplantation
- The Usefulness of the New ADAMTS-13 Activity Assay using a Fluorescence-quenching Substrate for the Diagnosis of Thrombotic Thrombocytopenic Purpura
- Association of Angiotensin-converting Enzyme Gene Polymorphism with the Disease Activity of Systemic Lupus Erythematosus in Korean Children