Kosin Med J.
2024 Dec;39(4):246-253. 10.7180/kmj.24.136.
Zonula occludens proteins and their impact on the cancer microenvironment
- Affiliations
-
- 1Department of Biomedical Sciences, Dong-A University College of Medicine, Busan, Korea
- 2Department of Parasitology and Genetics, Kosin University Gospel Hospital, Kosin University College of Medicine, Busan, Korea
- KMID: 2562807
- DOI: http://doi.org/10.7180/kmj.24.136
Abstract
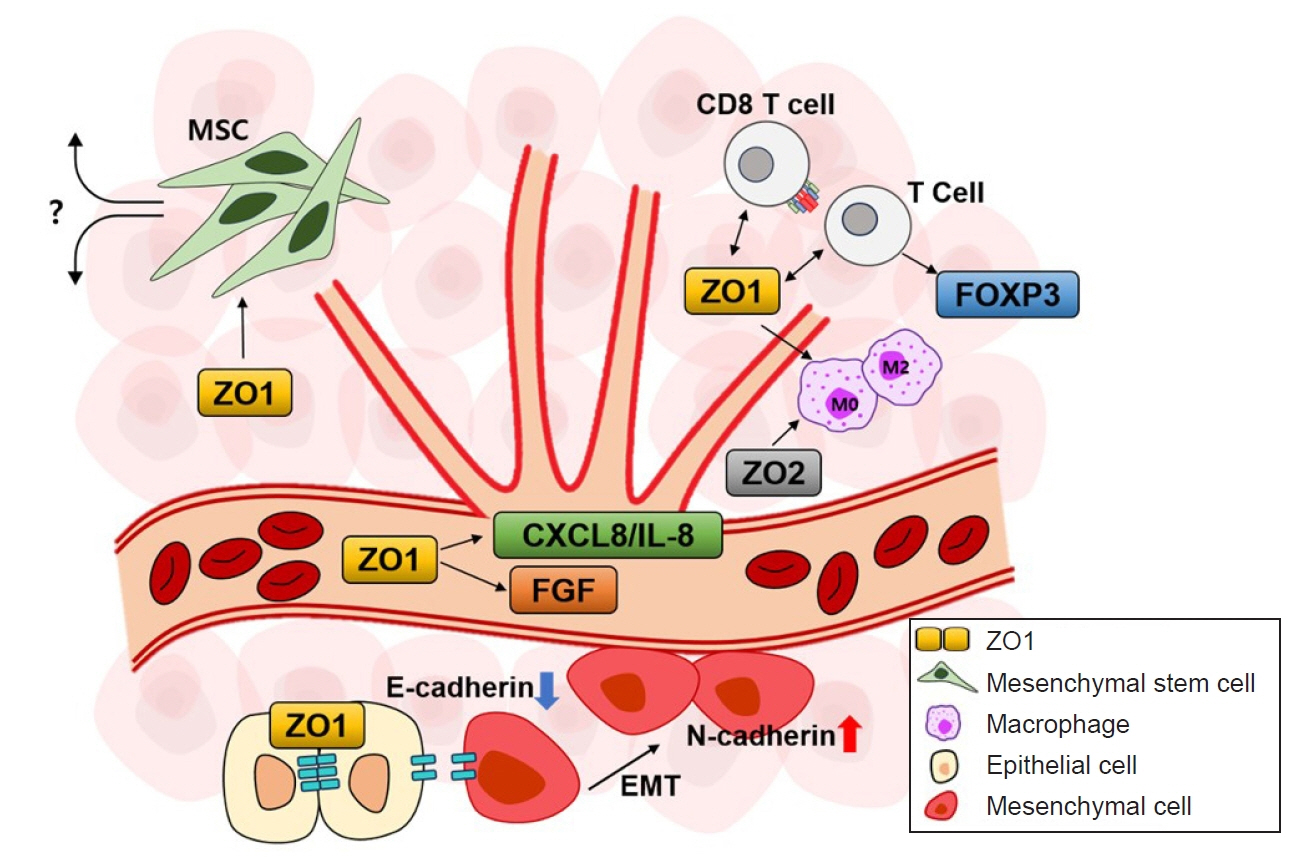
- Zonula occludens (ZO) proteins serve as scaffolding proteins that provide structural support at cell junctions and the cytoplasmic surface, acting as bridges between integral membrane proteins and the cytoskeleton. In addition to their structural functions, they also regulate cell growth and proliferation. Recent studies have shown that ZO proteins are involved in various diseases, including cancer. Specifically, ZO proteins influence the growth and development of cancer cells in the tumor microenvironment. These proteins perform various functions in the tumor microenvironment through processes such as angiogenesis, inflammatory responses, epithelial-mesenchymal transition, and interactions with mesenchymal stem cells. The mechanisms of these actions may vary depending on the type of cancer and environmental conditions. Ongoing research explores several signaling pathways involving ZO proteins. These insights suggest that new therapeutic approaches may be considered to slow down cancer growth and development within the tumor microenvironment. Despite continuing research on the cellular and in vivo roles of ZO proteins, the current understanding of how these signaling mechanisms function within the tumor microenvironment in vivo remains limited. In this review, we introduce the characteristics and regulatory mechanisms of ZO proteins in the cancer microenvironment, explore their potential to suppress cancer cell environments, and examine their roles in vivo.
Figure
Reference
-
References
1. Lee YC, Tsai KW, Liao JB, Kuo WT, Chang YC, Yang YF. High expression of tight junction protein 1 as a predictive biomarker for bladder cancer grade and staging. Sci Rep. 2022; 12:1496.
Article2. Martin TA, Watkins G, Mansel RE, Jiang WG. Loss of tight junction plaque molecules in breast cancer tissues is associated with a poor prognosis in patients with breast cancer. Eur J Cancer. 2004; 40:2717–25.
Article3. Zhang X, Wang L, Zhang H, Tu F, Qiang Y, Nie C. Decreased expression of ZO-1 is associated with tumor metastases in liver cancer. Oncol Lett. 2019; 17:1859–64.
Article4. Chen Y, Tang L, Ye X, Chen Y, Shan E, Han H, et al. Regulation of ZO-1 on β-catenin mediates sulforaphane suppressed colorectal cancer stem cell properties in colorectal cancer. Food Funct. 2022; 13:12363–70.
Article5. Chen B, Bu R, Xu X. Expression of tight junction proteins is altered in bladder cancer. Anal Cell Pathol (Amst). 2020; 2020:6341256.
Article6. Neyrinck-Leglantier D, Lesage J, Blacher S, Bonnomet A, Hunziker W, Noel A, et al. ZO-1 intracellular localization organizes immune response in non-small cell lung cancer. Front Cell Dev Biol. 2021; 9:749364.
Article7. Ram AK, Vairappan B. Role of zonula occludens in gastrointestinal and liver cancers. World J Clin Cases. 2022; 10:3647–61.
Article8. Baghban R, Roshangar L, Jahanban-Esfahlan R, Seidi K, Ebrahimi-Kalan A, Jaymand M, et al. Tumor microenvironment complexity and therapeutic implications at a glance. Cell Commun Signal. 2020; 18:59.
Article9. Kim HS, Lee SI, Choi YR, Kim J, Eun JW, Song KS, et al. GNAQ-regulated ZO-1 and ZO-2 act as tumor suppressors by modulating EMT potential and tumor-repressive microenvironment in lung cancer. Int J Mol Sci. 2023; 24:8801.
Article10. Lesage J, Suarez-Carmona M, Neyrinck-Leglantier D, Grelet S, Blacher S, Hunziker W, et al. Zonula occludens-1/NF-κB/CXCL8: a new regulatory axis for tumor angiogenesis. FASEB J. 2017; 31:1678–88.
Article11. Salvador E, Burek M, Forster CY. Tight junctions and the tumor microenvironment. Curr Pathobiol Rep. 2016; 4:135–45.
Article12. de Visser KE, Joyce JA. The evolving tumor microenvironment: from cancer initiation to metastatic outgrowth. Cancer Cell. 2023; 41:374–403.
Article13. Ko EJ, Kim DY, Kim MH, An H, Kim J, Jeong JY, et al. Functional analysis of membrane-associated scaffolding tight junction (TJ) proteins in tumorigenic characteristics of B16-F10 mouse melanoma cells. Int J Mol Sci. 2024; 25:833.
Article14. Kojima Y, Acar A, Eaton EN, Mellody KT, Scheel C, Ben-Porath I, et al. Autocrine TGF-beta and stromal cell-derived factor-1 (SDF-1) signaling drives the evolution of tumor-promoting mammary stromal myofibroblasts. Proc Natl Acad Sci U S A. 2010; 107:20009–14.15. Spaeth EL, Dembinski JL, Sasser AK, Watson K, Klopp A, Hall B, et al. Mesenchymal stem cell transition to tumor-associated fibroblasts contributes to fibrovascular network expansion and tumor progression. PLoS One. 2009; 4:e4992.
Article16. Dirat B, Bochet L, Dabek M, Daviaud D, Dauvillier S, Majed B, et al. Cancer-associated adipocytes exhibit an activated phenotype and contribute to breast cancer invasion. Cancer Res. 2011; 71:2455–65.
Article17. Tornavaca O, Chia M, Dufton N, Almagro LO, Conway DE, Randi AM, et al. ZO-1 controls endothelial adherens junctions, cell-cell tension, angiogenesis, and barrier formation. J Cell Biol. 2015; 208:821–38.
Article18. Lee TJ, Choi YH, Song KS. The PDZ motif peptide of ZO-1 attenuates Pseudomonas aeruginosa LPS-induced airway inflammation. Sci Rep. 2020; 10:19644.
Article19. Lee SH. Intestinal permeability regulation by tight junction: implication on inflammatory bowel diseases. Intest Res. 2015; 13:11–8.
Article20. Landy J, Ronde E, English N, Clark SK, Hart AL, Knight SC, et al. Tight junctions in inflammatory bowel diseases and inflammatory bowel disease associated colorectal cancer. World J Gastroenterol. 2016; 22:3117–26.
Article21. Ibrahim S, Zhu X, Luo X, Feng Y, Wang J. PIK3R3 regulates ZO-1 expression through the NF-kB pathway in inflammatory bowel disease. Int Immunopharmacol. 2020; 85:106610.
Article22. Seo H, Lee HC, Lee KC, Kim D, Kim J, Kang D, et al. PDZ Peptide of the ZO-1 protein significantly increases UTP-induced MUC8 anti-inflammatory mucin overproduction in human airway epithelial cells. Mol Cells. 2023; 46:700–9.
Article23. Ram AK, Pottakat B, Vairappan B. Increased systemic zonula occludens 1 associated with inflammation and independent biomarker in patients with hepatocellular carcinoma. BMC Cancer. 2018; 18:572.
Article24. Wu J, Zhou XJ, Sun X, Xia TS, Li XX, Shi L, et al. RBM38 is involved in TGF-β-induced epithelial-to-mesenchymal transition by stabilising zonula occludens-1 mRNA in breast cancer. Br J Cancer. 2017; 117:675–84.
Article25. Mauro L, Bartucci M, Morelli C, Ando S, Surmacz E. IGF-I receptor-induced cell-cell adhesion of MCF-7 breast cancer cells requires the expression of junction protein ZO-1. J Biol Chem. 2001; 276:39892–7.
Article26. Smalley KS, Brafford P, Haass NK, Brandner JM, Brown E, Herlyn M. Up-regulated expression of zonula occludens protein-1 in human melanoma associates with N-cadherin and contributes to invasion and adhesion. Am J Pathol. 2005; 166:1541–54.
Article27. Park A, Choi S, Do J, Kim Y, Kim KS, Koh E, et al. ZO-1 regulates the migration of mesenchymal stem cells in cooperation with α-catenin in response to breast tumor cells. Cell Death Discov. 2024; 10:19.
Article28. Hu S, Park J, Liu A, Lee J, Zhang X, Hao Q, et al. Mesenchymal stem cell microvesicles restore protein permeability across primary cultures of injured human lung microvascular endothelial cells. Stem Cells Transl Med. 2018; 7:615–24.
Article29. Hue CD, Cho FS, Cao S, Dale Bass CR, Meaney DF, Morrison B 3rd. Dexamethasone potentiates in vitro blood-brain barrier recovery after primary blast injury by glucocorticoid receptor-mediated upregulation of ZO-1 tight junction protein. J Cereb Blood Flow Metab. 2015; 35:1191–8.
Article30. Lima WR, Parreira KS, Devuyst O, Caplanusi A, N’kuli F, Marien B, et al. ZONAB promotes proliferation and represses differentiation of proximal tubule epithelial cells. J Am Soc Nephrol. 2010; 21:478–88.
Article31. Georgiadis A, Tschernutter M, Bainbridge JW, Balaggan KS, Mowat F, West EL, et al. The tight junction associated signalling proteins ZO-1 and ZONAB regulate retinal pigment epithelium homeostasis in mice. PLoS One. 2010; 5:e15730.
Article32. Balda MS, Garrett MD, Matter K. The ZO-1-associated Y-box factor ZONAB regulates epithelial cell proliferation and cell density. J Cell Biol. 2003; 160:423–32.
Article33. Christensen NR, Calyseva J, Fernandes EF, Luchow S, Clemmensen LS, Haugaard-Kedstrom LM, et al. PDZ domains as drug targets. Adv Ther (Weinh). 2019; 2:1800143.
Article34. Reichert M, Muller T, Hunziker W. The PDZ domains of zonula occludens-1 induce an epithelial to mesenchymal transition of Madin-Darby canine kidney I cells: evidence for a role of beta-catenin/Tcf/Lef signaling. J Biol Chem. 2000; 275:9492–500.35. Utepbergenov DI, Fanning AS, Anderson JM. Dimerization of the scaffolding protein ZO-1 through the second PDZ domain. J Biol Chem. 2006; 281:24671–7.
Article36. Sordillo PP, Helson L. Curcumin and cancer stem cells: curcumin has asymmetrical effects on cancer and normal stem cells. Anticancer Res. 2015; 35:599–614.37. Shen YA, Lin CH, Chi WH, Wang CY, Hsieh YT, Wei YH, et al. Resveratrol impedes the stemness, epithelial-mesenchymal transition, and metabolic reprogramming of cancer stem cells in nasopharyngeal carcinoma through p53 activation. Evid Based Complement Alternat Med. 2013; 2013:590393.
Article38. Carrasco-Pozo C, Tan KN, Avery VM. Hemin prevents increased glycolysis in macrophages upon activation: protection by microbiota-derived metabolites of polyphenols. Antioxidants (Basel). 2020; 9:1109.
Article39. Tian S, Guo R, Wei S, Kong Y, Wei X, Wang W, et al. Curcumin protects against the intestinal ischemia-reperfusion injury: involvement of the tight junction protein ZO-1 and TNF-α related mechanism. Korean J Physiol Pharmacol. 2016; 20:147–52.
Article40. Luo Y, Yu X, Zhao P, Huang J, Huang X. Effects of resveratrol on tight junction proteins and the notch1 pathway in an HT-29 cell model of inflammation induced by lipopolysaccharide. Inflammation. 2022; 45:2449–64.
Article41. Chen X, Zhao M, Huang J, Li Y, Wang S, Harrington CA, et al. microRNA-130a suppresses breast cancer cell migration and invasion by targeting FOSL1 and upregulating ZO-1. J Cell Biochem. 2018; 119:4945–56.
Article42. Ghosh D, Dutta A, Kashyap A, Upmanyu N, Datta S. PLP2 drives collective cell migration via ZO-1-mediated cytoskeletal remodeling at the leading edge in human colorectal cancer cells. J Cell Sci. 2021; 134:jcs253468.
Article43. Haas AJ, Zihni C, Krug SM, Maraspini R, Otani T, Furuse M, et al. ZO-1 guides tight junction assembly and epithelial morphogenesis via cytoskeletal tension-dependent and -independent functions. Cells. 2022; 11:3775.
Article44. Xu J, Kausalya PJ, Ong AG, Goh CM, Mohamed Ali S, Hunziker W. ZO-2/Tjp2 suppresses Yap and Wwtr1/Taz-mediated hepatocyte to cholangiocyte transdifferentiation in the mouse liver. NPJ Regen Med. 2022; 7:55.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Expression of Osteopontin, ZO-1 and E-cadherin in Adenoma and Adenocarcinoma of the Colon
- Effects of Interleukin-13 and Montelukast on the Expression of Zonula Occludens-1 in Human Podocytes
- Chlorogenic Acid Decreases Retinal Vascular Hyperpermeability in Diabetic Rat Model
- Expression of claudin-1, claudin-4 and zonula occludens-1 in cervical intraepithelial neoplasia and invasive squamous cell carcinoma
- Hypoxia Increases Epithelial Permeability in Human Nasal Epithelia