Ann Hepatobiliary Pancreat Surg.
2024 Nov;28(4):458-465. 10.14701/ahbps.24-086.
Internal and external validation of indocyanine green plasma disappearance rate to discard liver grafts before procurement
- Affiliations
-
- 1Liver Transplant Unit, Hospital General Universitario Gregorio Marañón, Madrid, Spain
- 2Department of General and Digestive Surgery, Hospital General Universitario Gregorio Marañón, Madrid, Spain
- 3Hospital Universitario Río Hortega, Valladolid, Spain
- 4Liver Transplant and HPB Surgery Unit, Department of General and Digestive Surgery, Hospital Virgen de las Nieves, Granada, Spain
- 5Hospital Universitario Nuestra Señora de Candelaria, Santa Cruz de Tenerife, Spain
- 6Department of Medicine, Faculty of Medicine, Universidad Complutense de Madrid, Madrid, Spain
- KMID: 2561578
- DOI: http://doi.org/10.14701/ahbps.24-086
Abstract
- Backgrounds/Aims
Thirty percent of liver grafts in donors after brain death (DBD) in Spain are rejected by procurement surgeons owing to marginal graft quality. Poor donor indocyanine green (ICG) clearance has been associated with graft discard and malfunction. This study aimed to internally and externally validate the predictive value of ICG-plasma disappearance rate (ICG-PDR) to reject grafts before donation and set a cut-off to avoid missing any potential effective donors.
Methods
Between March 2017 and August 2023, ICG clearance test was performed immediately before procurement in 71 DBD. The surgeon was blinded to test results. Univariate and multivariate analyses were performed to detect independent predictors of graft discard. Discrimination and calibration of predictors were assessed and a cut-off with 100% specificity was set. External validation was performed on 17 donors evaluated by three other transplantation teams.
Results
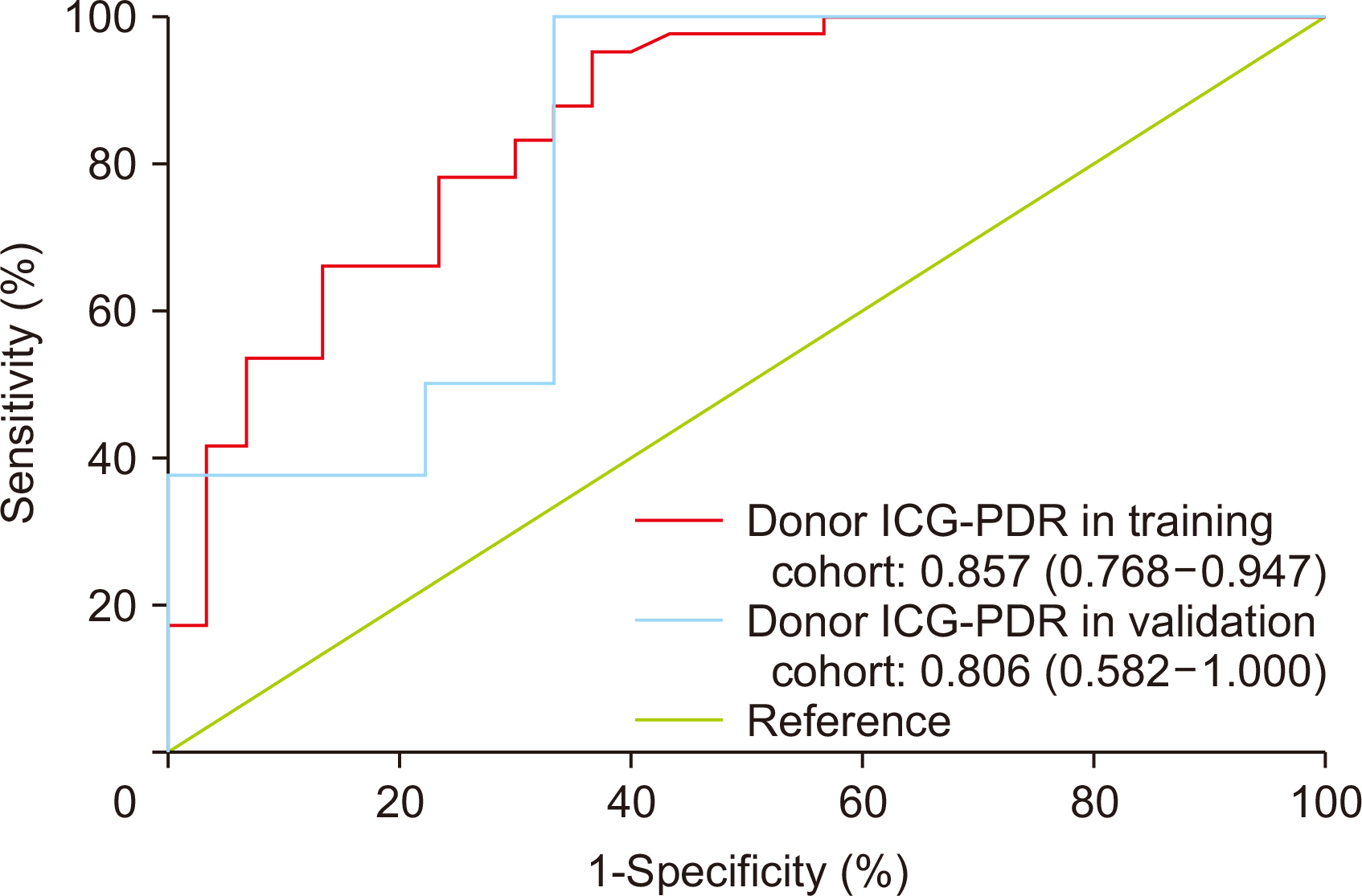
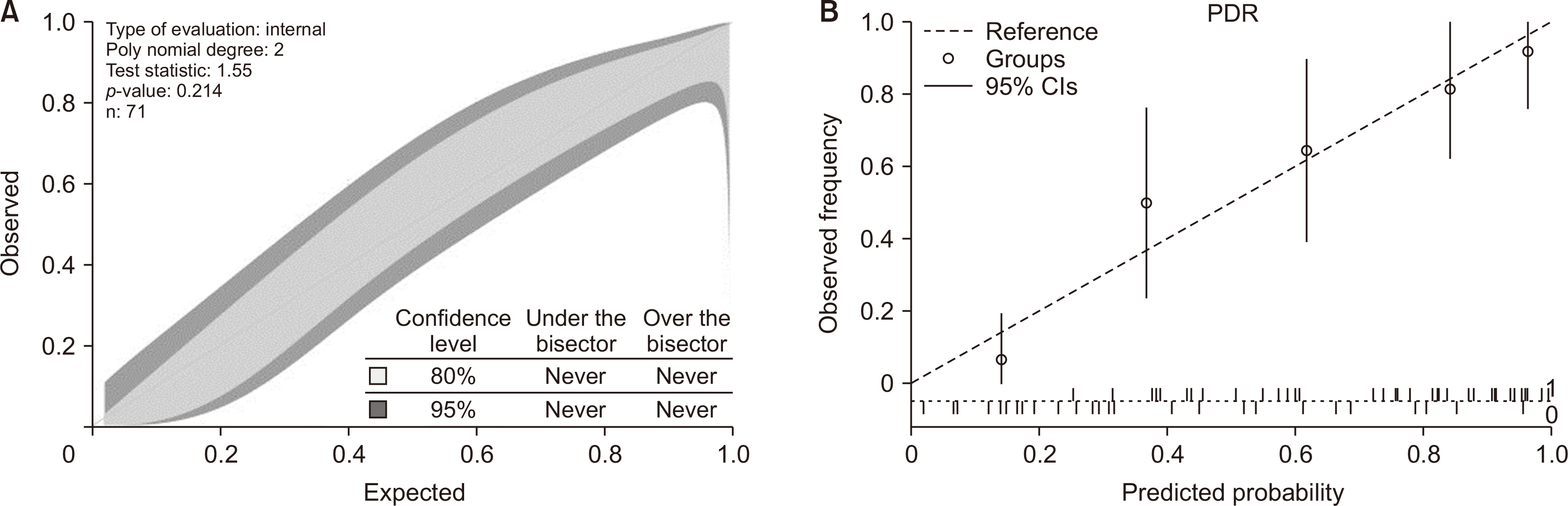
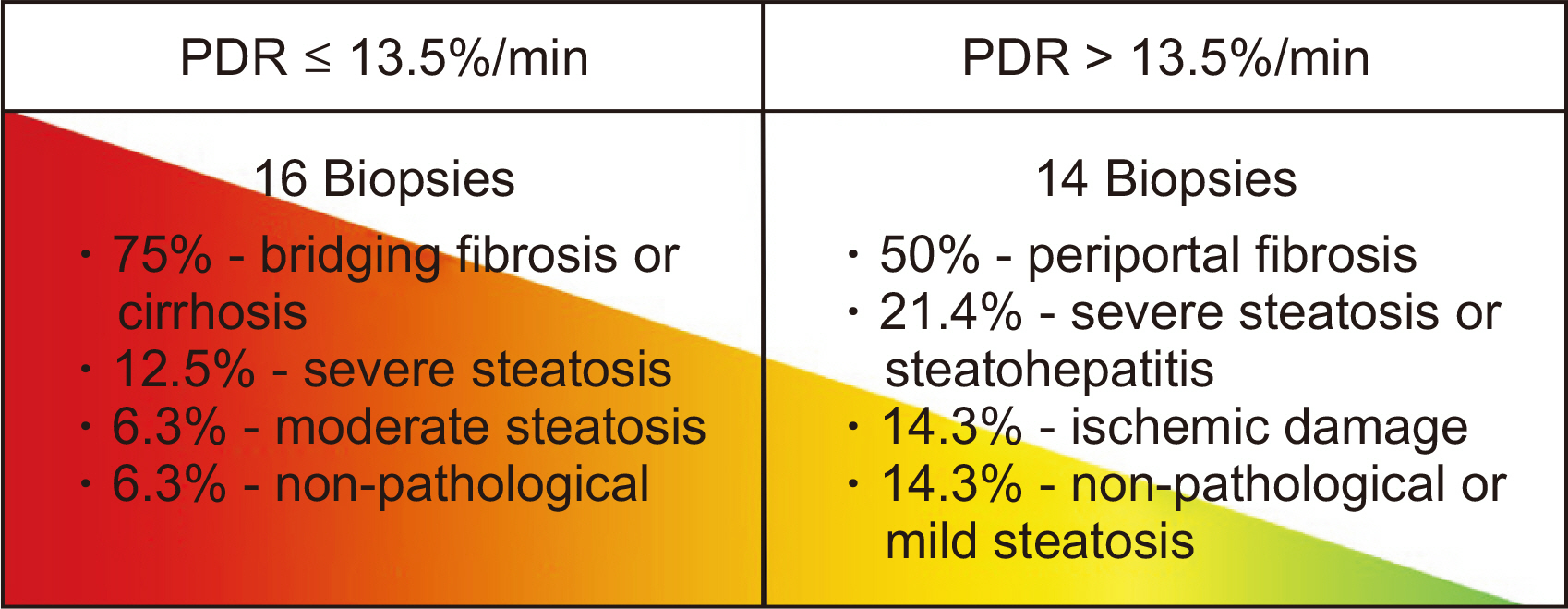
In the training cohort, 30 of 71 grafts were discarded for transplantation. ICG-PDR was the only donor variable independently associated with graft discard. The area under receiver operating characteristic curve for ICG-PDR was 0.875 (95% confidence interval: 0.768–0.947) and good calibration was observed. Below a PDR of 13.5%/min, no graft was accepted for transplantation. These results were successfully validated using the external cohort of donors.
Conclusions
ICG clearance test performed in DBD was internally and externally validated to predict liver graft discard. It could be used as a screening tool before donation to avoid unnecessary costs of travel and human resources.
Figure
Reference
-
References
1. Organización Nacional de Trasplantes (ONT). Actividad de donación y trasplante hepático España 2022 [Internet]. ONT;2022. Available from: https://www.ont.es/wp-content/uploads/2023/06/DONACION-Y-TRASPLANTE-HEPATICO-2022.pdf. cited 2024 Jan 23.2. Ali Deeb A, Settmacher U, Fritsch J, Dondorf F, Rohland O, Rauchfuß F. 2023; Postoperative outcomes in 415 patients following liver transplantation using extended donor criteria: a study from a single center in Germany. Ann Transplant. 28:e939060. DOI: 10.12659/AOT.939060.3. Mangus RS, Borup TC, Popa S, Saxena R, Cummings O, Tector AJ. 2014; Utility of pre-procurement bedside liver biopsy in the deceased extended-criteria liver donor. Clin Transplant. 28:1358–1364. DOI: 10.1111/ctr.12461. PMID: 25203789.4. Oliver JB, Machineni P, Bongu A, Patel T, Nespral J, Kadric C, et al. 2018; Liver biopsy in assessment of extended criteria donors. Liver Transpl. 24:182–191. DOI: 10.1002/lt.24947. PMID: 28941082.5. Saadeh S, Younossi ZM, Remer EM, Gramlich T, Ong JP, Hurley M, et al. 2002; The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology. 123:745–750. DOI: 10.1053/gast.2002.35354. PMID: 12198701.6. Nishio T, Taura K, Koyama Y, Ishii T, Hatano E. 2023; Current status of preoperative risk assessment for posthepatectomy liver failure in patients with hepatocellular carcinoma. Ann Gastroenterol Surg. 7:871–886. DOI: 10.1002/ags3.12692. PMID: 37927928. PMCID: PMC10623981.7. Olmedilla L, Lisbona CJ, Pérez-Peña JM, López-Baena JA, Garutti I, Salcedo M, et al. 2016; Early measurement of indocyanine green clearance accurately predicts short-term outcomes after liver transplantation. Transplantation. 100:613–620. DOI: 10.1097/TP.0000000000000980. PMID: 26569066.8. Koneru B, Leevy CB, Klein KM, Zweil P, Wilson DJ. 1993; Indocyanine green clearance in the evaluation of donor livers. Transplant Proc. 25:1919–1920.9. Tang Y, Han M, Chen M, Wang X, Ji F, Zhao Q, et al. 2017; Donor indocyanine green clearance test predicts graft quality and early graft prognosis after liver transplantation. Dig Dis Sci. 62:3212–3220. DOI: 10.1007/s10620-017-4765-x. PMID: 28932926.10. Zarrinpar A, Lee C, Noguchi E, Yersiz H, Agopian VG, Kaldas FM, et al. 2015; A rapid, reproducible, noninvasive predictor of liver graft survival. J Surg Res. 197:183–190. DOI: 10.1016/j.jss.2015.03.093. PMID: 25940156. PMCID: PMC5025251.11. Asencio JM, Cortese S, López Baena JA, Olmedilla L, Pérez Peña JM, Salcedo MM, et al. 2020; Evaluation of plasma disappearance rate indocyanine green clearance as a predictor of liver graft rejection in donor brain death. Transplant Proc. 52:1472–1476. DOI: 10.1016/j.transproceed.2020.01.084. PMID: 32217011.12. Olthoff KM, Kulik L, Samstein B, Kaminski M, Abecassis M, Emond J, et al. 2010; Validation of a current definition of early allograft dysfunction in liver transplant recipients and analysis of risk factors. Liver Transpl. 16:943–949. DOI: 10.1002/lt.22091. PMID: 20677285.13. Moons KG, Altman DG, Reitsma JB, Ioannidis JP, Macaskill P, Steyerberg EW, et al. 2015; Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): explanation and elaboration. Ann Intern Med. 162:W1–W73. DOI: 10.7326/M14-0698. PMID: 25560730.14. Braat AE, Blok JJ, Putter H, Adam R, Burroughs AK, Rahmel AO, et al. 2012; The Eurotransplant donor risk index in liver transplantation: ET-DRI. Am J Transplant. 12:2789–2796. DOI: 10.1111/j.1600-6143.2012.04195.x. PMID: 22823098.15. Wesslau C, Kruger R, May G. 1994; Clinical investigations using indocyanine green clearance for evaluation of liver function in organ donors. Transplantology. 5:1–3.16. Janssen MW, Druckrey-Fiskaaen KT, Omidi L, Sliwinski G, Thiele C, Donaubauer B, et al. 2010; Indocyanine green R15 ratio depends directly on liver perfusion flow rate. J Hepatobiliary Pancreat Sci. 17:180–185. DOI: 10.1007/s00534-009-0160-0. PMID: 19760140.17. Sakka SG. 2018; Assessment of liver perfusion and function by indocyanine green in the perioperative setting and in critically ill patients. J Clin Monit Comput. 32:787–796. DOI: 10.1007/s10877-017-0073-4. PMID: 29039062.18. Parente A, Tirotta F, Pini A, Eden J, Dondossola D, Manzia TM, et al. 2023; Machine perfusion techniques for liver transplantation - a meta-analysis of the first seven randomized-controlled trials. J Hepatol. 79:1201–1213. DOI: 10.1016/j.jhep.2023.05.027. PMID: 37302578.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of the Indocyanine Green Clearance Test Using Conventional Blood Sampling and Finger Monitoring Methods
- A Case of Rotor Syndrome
- Ablative Fractional Radiofrequency Combined with Sonophoresis Increases Skin Penetration of Indocyanine Green
- Peritoneoscopic Examination of the Liver Disease Stained by Intravenous Injection of Indocyanine Green
- Utility of indocyanine green for diagnosing peritoneal dialysis-related hydrothorax