Ewha Med J.
2024 Oct;47(4):e58. 10.12771/emj.2024.e58.
The histopathological and molecular heterogeneity of hepatocellular carcinoma: a narrative review
- Affiliations
-
- 1Department of Pathology, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea
- KMID: 2561406
- DOI: http://doi.org/10.12771/emj.2024.e58
Abstract
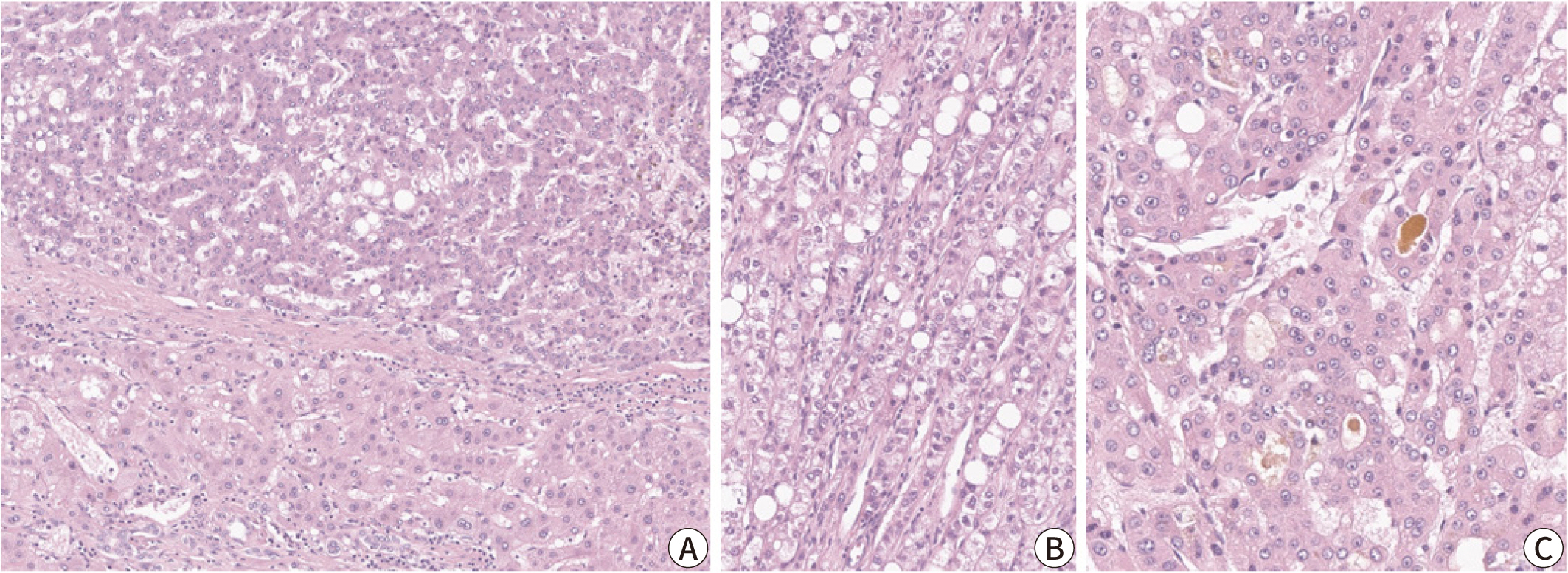
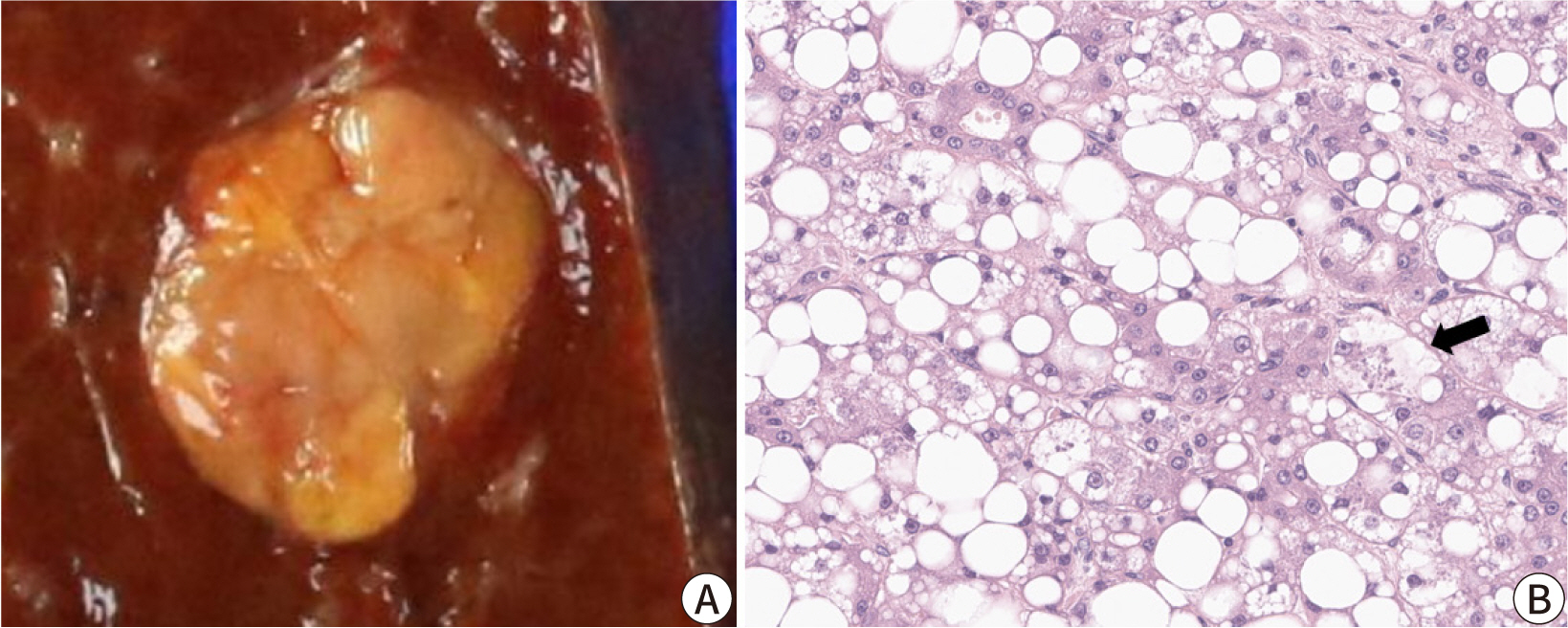
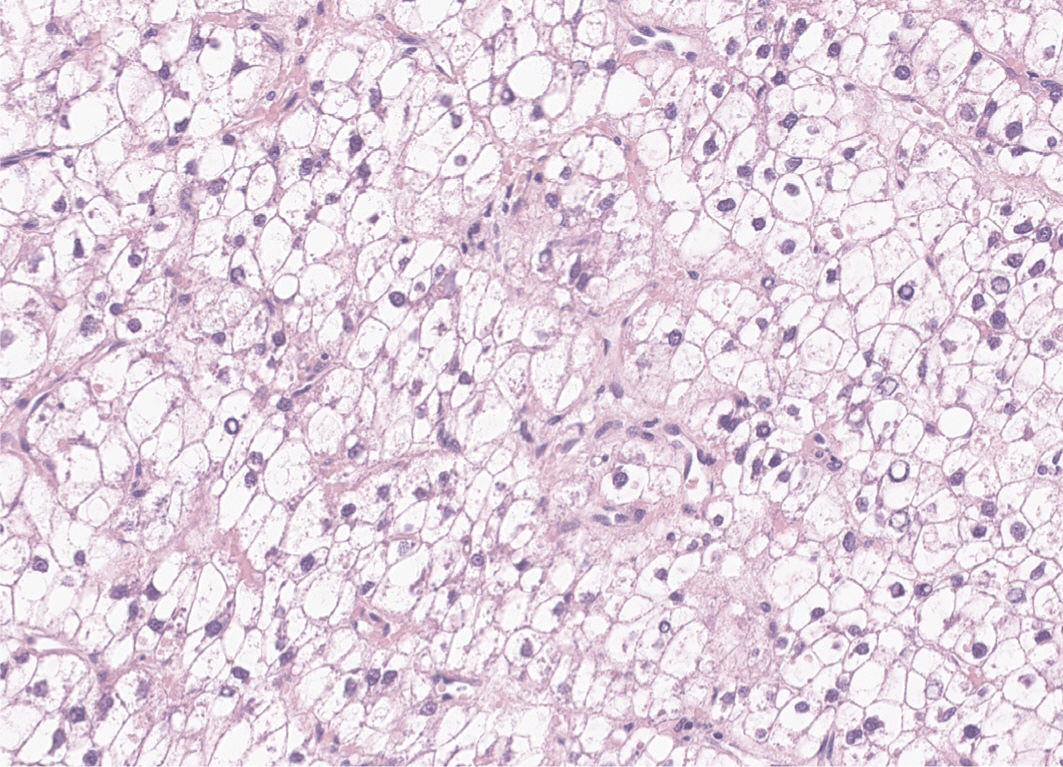
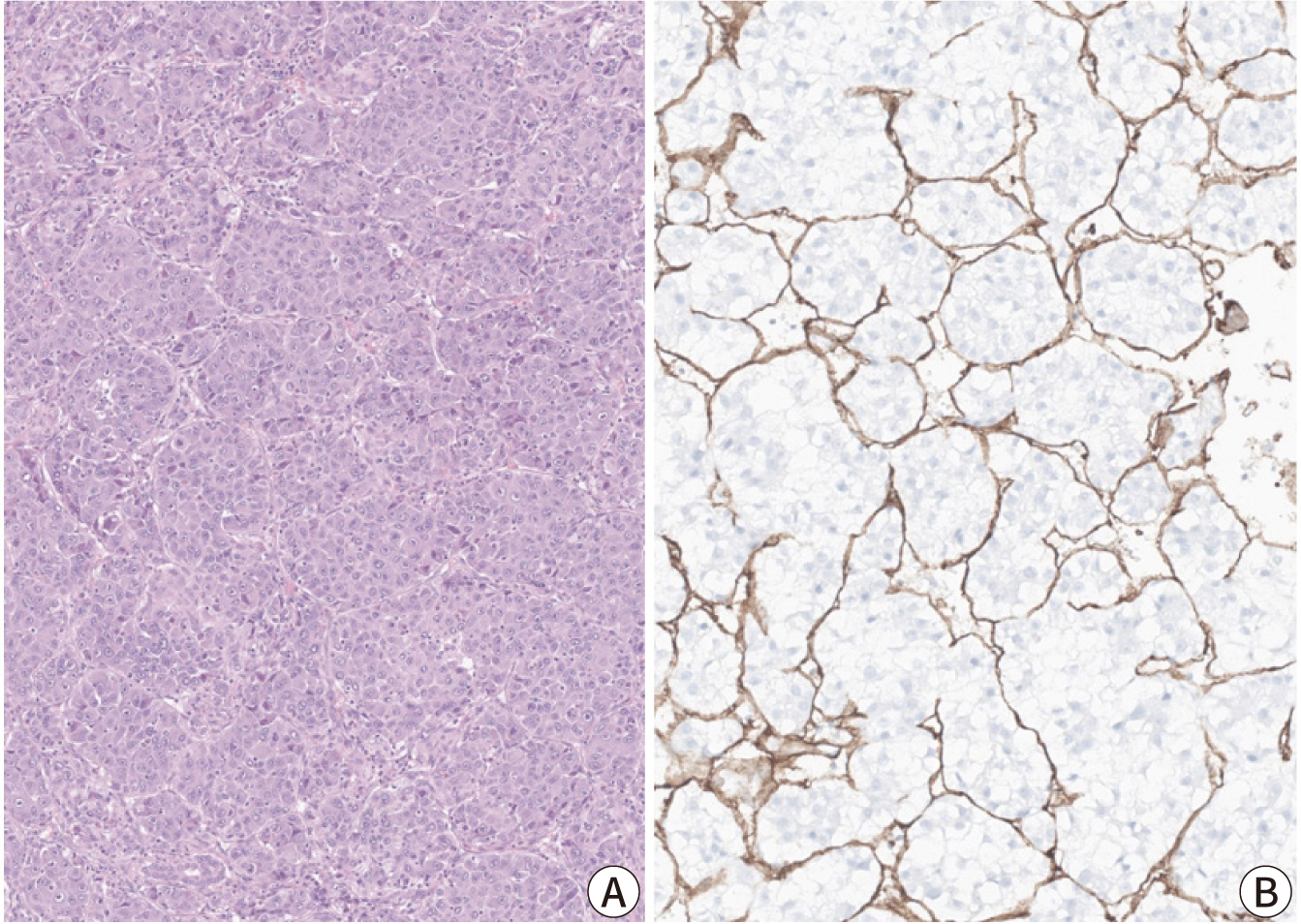
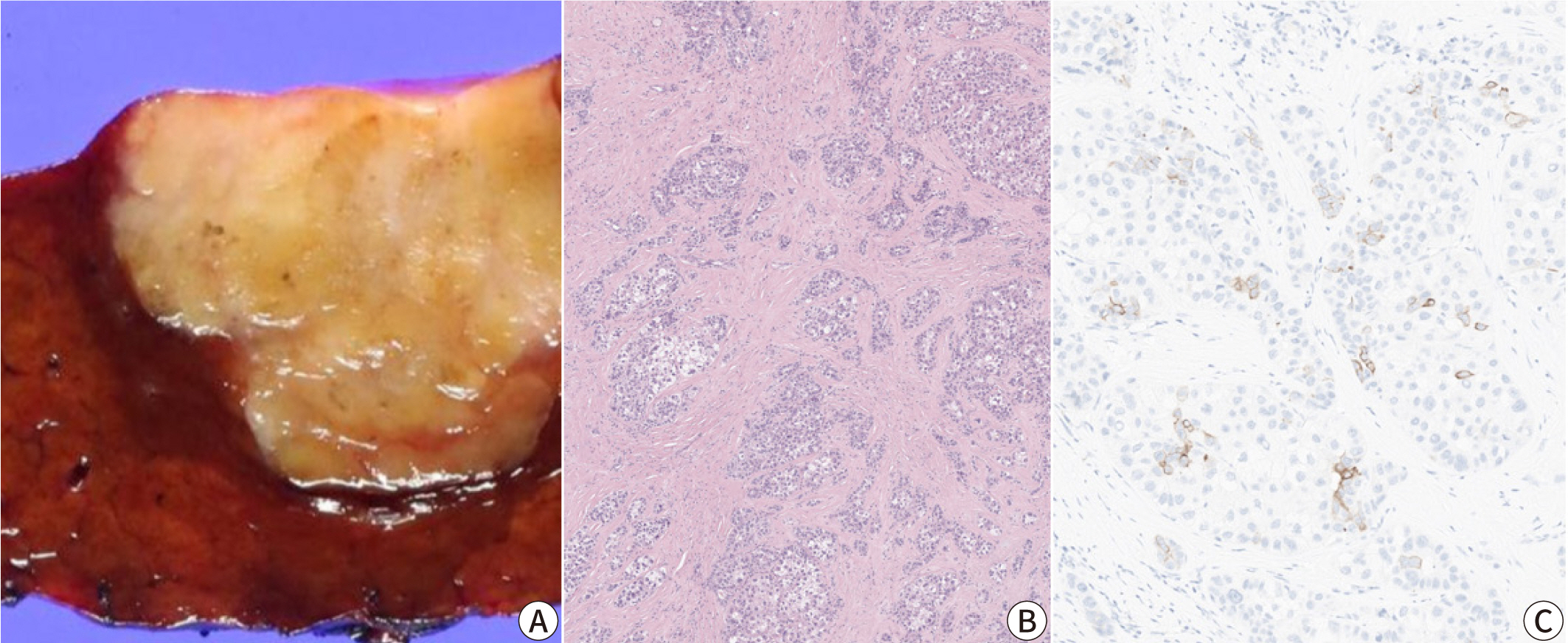
- Hepatocellular carcinoma (HCC) remains a leading cause of cancer-related deaths worldwide, with poor clinical outcomes due to challenges in early detection and limited efficacy of current treatments such as receptor tyrosine kinase inhibitors and immunotherapy. HCC exhibits significant heterogeneity at both histopathological and molecular levels, complicating its management but offering potential for personalized therapeutic approaches. This review outlines the morpho-molecular heterogeneity of HCC and summarizes various histological subtypes, including steatohepatitic, clear cell, macrotrabecular-massive, scirrhous, lymphocyte-rich, and fibrolamellar HCCs. Each subtype possesses distinct clinical, histological, and molecular features; for instance, steatohepatitic HCC is associated with metabolic dysfunction and shows IL-6/JAK/STAT activation, while clear cell HCCs often have IDH1 mutations and favorable prognosis. The macrotrabecular-massive subtype is linked to poor outcomes and TP53 mutations, whereas scirrhous HCCs express stemness markers and have TSC1/TSC2 mutations. Lymphocyte-rich HCCs are characterized by immune cell infiltration and better prognosis. CTNNB1-mutated HCCs show specific morphological features and may benefit from targeted therapies. Understanding these subtypes and associated molecular alterations is crucial for developing effective diagnostic and therapeutic strategies, including potential predictive biomarkers and personalized treatments. Additionally, the identification of patterns like vessels-encapsulatingtumor-clusters offers prognostic implications and may guide therapeutic decisions. Recent molecular studies have enhanced our comprehension of HCC heterogeneity, laying the groundwork for more personalized approaches. Pathologists play a vital role in recognizing these subtypes, aiding in prognosis prediction and treatment planning. Advances in digital pathology and artificial intelligence may further facilitate biomarker research, ultimately improving patient outcomes in HCC management.
Keyword
Figure
Reference
-
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018; 68(6):394–424. DOI: 10.3322/caac.21492. PMID: 30207593.
Article2. Kim DY. Changing etiology and epidemiology of hepatocellular carcinoma: Asia and worldwide. J Liver Cancer. 2024; 24(1):62–70. DOI: 10.17998/jlc.2024.03.13. PMID: 38523466. PMCID: PMC10990659.3. Daher D, Dahan KSE, Singal AG. Non-alcoholic fatty liver disease-related hepatocellular carcinoma. J Liver Cancer. 2023; 23(1):127–142. DOI: 10.17998/jlc.2022.12.30. PMID: 37384032. PMCID: PMC10202236.4. Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012; 379(9822):1245–1255. DOI: 10.1016/S0140-6736(11)61347-0. PMID: 22353262.
Article5. Gordan JD, Kennedy EB, Abou-Alfa GK, Beg MS, Brower ST, Gade TP, et al. Systemic therapy for advanced hepatocellular carcinoma: ASCO guideline. J Clin Oncol. 2020; 38(36):4317–4345. DOI: 10.1200/JCO.20.02672. PMID: 33197225.6. Doycheva I, Thuluvath PJ. Systemic therapy for advanced hepatocellular carcinoma: an update of a rapidly evolving field. J Clin Exp Hepatol. 2019; 9(5):588–596. DOI: 10.1016/j.jceh.2019.07.012. PMID: 31695249. PMCID: PMC6823698.
Article7. Song YG, Yoo JJ, Kim SG, Kim YS. Complications of immunotherapy in advanced hepatocellular carcinoma. J Liver Cancer. 2024; 24(1):9–16. DOI: 10.17998/jlc.2023.11.21. PMID: 38018074. PMCID: PMC10990673.8. Burt AD, Ferrell LD, Hübscher SG. MacSween's pathology of the liver. Amsterdam: Elsevier Health Sciences;2022.9. Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, et al. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020; 76(2):182–188. DOI: 10.1111/his.13975. PMID: 31433515. PMCID: PMC7003895.
Article10. Edmondson HA, Steiner PE. Primary carcinoma of the liver: a study of 100 cases among 48,900 necropsies. Cancer. 1954; 7(3):462–503. DOI: 10.1002/1097-0142(195405)7:3<462::AID-CNCR2820070308>3.0.CO;2-E. PMID: 13160935.
Article11. Martins-Filho SN, Paiva C, Azevedo RS, Alves VAF. Histological grading of hepatocellular carcinoma: a systematic review of literature. Front Med. 2017; 4:193. DOI: 10.3389/fmed.2017.00193. PMID: 29209611. PMCID: PMC5701623.12. Lee JS. The mutational landscape of hepatocellular carcinoma. Clin Mol Hepatol. 2015; 21(3):220–229. DOI: 10.3350/cmh.2015.21.3.220. PMID: 26523267. PMCID: PMC4612282.
Article13. The Cancer Genome Atlas Research Network. Comprehensive and integrative genomic characterization of hepatocellular carcinoma. Cell. 2017; 169(7):1327–1341.E23.14. Calderaro J, Couchy G, Imbeaud S, Amaddeo G, Letouzé E, Blanc JF, et al. Histological subtypes of hepatocellular carcinoma are related to gene mutations and molecular tumour classification. J Hepatol. 2017; 67(4):727–738. DOI: 10.1016/j.jhep.2017.05.014. PMID: 28532995.
Article15. Lee JS, Heo J, Libbrecht L, Chu IS, Kaposi-Novak P, Calvisi DF, et al. A novel prognostic subtype of human hepatocellular carcinoma derived from hepatic progenitor cells. Nat Med. 2006; 12(4):410–416. DOI: 10.1038/nm1377. PMID: 16532004.
Article16. Boyault S, Rickman DS, de Reyniès A, Balabaud C, Rebouissou S, Jeannot E, et al. Transcriptome classification of HCC is related to gene alterations and to new therapeutic targets. Hepatology. 2007; 45(1):42–52. DOI: 10.1002/hep.21467. PMID: 17187432.
Article17. Chiang DY, Villanueva A, Hoshida Y, Peix J, Newell P, Minguez B, et al. Focal gains of VEGFA and molecular classification of hepatocellular carcinoma. Cancer Res. 2008; 68(16):6779–6788. DOI: 10.1158/0008-5472.CAN-08-0742. PMID: 18701503. PMCID: PMC2587454.
Article18. Hoshida Y, Nijman SMB, Kobayashi M, Chan JA, Brunet JP, Chiang DY, et al. Integrative transcriptome analysis reveals common molecular subclasses of human hepatocellular carcinoma. Cancer Res. 2009; 69(18):7385–7392. DOI: 10.1158/0008-5472.CAN-09-1089. PMID: 19723656. PMCID: PMC3549578.
Article19. Sia D, Jiao Y, Martinez-Quetglas I, Kuchuk O, Villacorta-Martin C, Castro de Moura M, et al. Identification of an immune-specific class of hepatocellular carcinoma, based on molecular features. Gastroenterology. 2017; 153(3):812–826. DOI: 10.1053/j.gastro.2017.06.007. PMID: 28624577.
Article20. Audard V, Grimber G, Elie C, Radenen B, Audebourg A, Letourneur F, et al. Cholestasis is a marker for hepatocellular carcinomas displaying β-catenin mutations. J Pathol. 2007; 212(3):345–352. DOI: 10.1002/path.2169. PMID: 17487939.
Article21. Salomao M, Yu WM, Brown RS Jr, Emond JC, Lefkowitch JH. Steatohepatitic hepatocellular carcinoma (SH-HCC): a distinctive histological variant of HCC in hepatitis C virus-related cirrhosis with associated NAFLD/NASH. Am J Surg Pathol. 2010; 34(11):1630–1636. DOI: 10.1097/PAS.0b013e3181f31caa. PMID: 20975341.22. Bannasch P, Ribback S, Su Q, Mayer D. Clear cell hepatocellular carcinoma: origin, metabolic traits and fate of glycogenotic clear and ground glass cells. Hepatobiliary Pancreatic Dis Int. 2017; 16(6):570–594. DOI: 10.1016/S1499-3872(17)60071-7. PMID: 29291777.
Article23. Li T, Fan J, Qin LX, Zhou J, Sun HC, Qiu SJ, et al. Risk factors, prognosis, and management of early and late intrahepatic recurrence after resection of primary clear cell carcinoma of the liver. Ann Surg Oncol. 2011; 18(7):1955–1963. DOI: 10.1245/s10434-010-1540-z. PMID: 21240562.
Article24. Lee JH, Shin DH, Park WY, Shin N, Kim A, Lee HJ, et al. IDH1 R132C mutation is detected in clear cell hepatocellular carcinoma by pyrosequencing. World J Surg Oncol. 2017; 15(1):82. DOI: 10.1186/s12957-017-1144-1. PMID: 28403884. PMCID: PMC5389153.25. Jeon Y, Benedict M, Taddei T, Jain D, Zhang X. Macrotrabecular hepatocellular carcinoma: an aggressive subtype of hepatocellular carcinoma. Am J Surg Pathol. 2019; 43(7):943–948. DOI: 10.1097/PAS.0000000000001289. PMID: 31135484.26. Kumar D, Hafez O, Jain D, Zhang X. Can primary hepatocellular carcinoma histomorphology predict extrahepatic metastasis? Hum Pathol. 2021; 113:39–46. DOI: 10.1016/j.humpath.2021.04.008. PMID: 33905775.
Article27. Renne SL, Woo HY, Allegra S, Rudini N, Yano H, Donadon M, et al. Vessels encapsulating tumor clusters (VETC) is a powerful predictor of aggressive hepatocellular carcinoma. Hepatology. 2020; 71(1):183–195. DOI: 10.1002/hep.30814. PMID: 31206715.
Article28. Fang JH, Xu L, Shang LR, Pan CZ, Ding J, Tang YQ, et al. Vessels that encapsulate tumor clusters (VETC) pattern is a predictor of sorafenib benefit in patients with hepatocellular carcinoma. Hepatology. 2019; 70(3):824–839. DOI: 10.1002/hep.30366. PMID: 30506570.
Article29. Kurogi M, Nakashima O, Miyaaki H, Fujimoto M, Kojiro M. Clinicopathological study of scirrhous hepatocellular carcinoma. J Gastroenterol Hepatol. 2006; 21(9):1470–1477. DOI: 10.1111/j.1440-1746.2006.04372.x. PMID: 16911695.30. Seok JY, Na DC, Woo HG, Roncalli M, Kwon SM, Yoo JE, et al. A fibrous stromal component in hepatocellular carcinoma reveals a cholangiocarcinoma-like gene expression trait and epithelial-mesenchymal transition. Hepatology. 2012; 55(6):1776–1786. DOI: 10.1002/hep.25570. PMID: 22234953.
Article31. Matsuura S, Aishima S, Taguchi K, Asayama Y, Terashi T, Honda H, et al. 'Scirrhous' type hepatocellular carcinomas: a special reference to expression of cytokeratin 7 and hepatocyte paraffin 1. Histopathology. 2005; 47(4):382–390. DOI: 10.1111/j.1365-2559.2005.02230.x. PMID: 16178893.
Article32. Chan AWH, Tong JHM, Pan Y, Chan SL, Wong GLH, Wong VWS, et al. Lymphoepithelioma-like hepatocellular carcinoma: an uncommon variant of hepatocellular carcinoma with favorable outcome. Am J Surg Pathol. 2015; 39(3):304–312. DOI: 10.1097/PAS.0000000000000376. PMID: 25675010.33. Calderaro J, Rousseau B, Amaddeo G, Mercey M, Charpy C, Costentin C, et al. Programmed death ligand 1 expression in hepatocellular carcinoma: relationship with clinical and pathological features. Hepatology. 2016; 64(6):2038–2046. DOI: 10.1002/hep.28710. PMID: 27359084.
Article34. Nishida N, Sakai K, Morita M, Aoki T, Takita M, Hagiwara S, et al. Association between genetic and immunological background of hepatocellular carcinoma and expression of programmed cell death-1. Liver Cancer. 2020; 9(4):426–439. DOI: 10.1159/000506352. PMID: 32999869. PMCID: PMC7506256.
Article35. Chan AWH, Zhang Z, Chong CCN, Tin EKY, Chow C, Wong N. Genomic landscape of lymphoepithelioma-like hepatocellular carcinoma. J Pathol. 2019; 249(2):166–172. DOI: 10.1002/path.5313. PMID: 31168847.36. Torbenson M. Fibrolamellar carcinoma: 2012 update. Scientifica. 2012; 2012:743790. DOI: 10.6064/2012/743790. PMID: 24278737. PMCID: PMC3820672.
Article37. El-Serag HB, Davila JA. Is fibrolamellar carcinoma different from hepatocellular carcinoma? A US population-based study. Hepatology. 2004; 39(3):798–803. DOI: 10.1002/hep.20096. PMID: 14999699.
Article38. Graham RP, Yeh MM, Lam-Himlin D, Roberts LR, Terracciano L, Cruise MW, et al. Molecular testing for the clinical diagnosis of fibrolamellar carcinoma. Mod Pathol. 2018; 31(1):141–149. DOI: 10.1038/modpathol.2017.103. PMID: 28862261. PMCID: PMC5758901.
Article39. Gougelet A, Torre C, Veber P, Sartor C, Bachelot L, Denechaud PD, et al. T-cell factor 4 and β-catenin chromatin occupancies pattern zonal liver metabolism in mice. Hepatology. 2014; 59(6):2344–2357. DOI: 10.1002/hep.26924. PMID: 24214913.
Article40. Monga SP. β-Catenin signaling and roles in liver homeostasis, injury, and tumorigenesis. Gastroenterology. 2015; 148(7):1294–1310. DOI: 10.1053/j.gastro.2015.02.056. PMID: 25747274. PMCID: PMC4494085.
Article41. Ueno A, Masugi Y, Yamazaki K, Komuta M, Effendi K, Tanami Y, et al. OATP1B3 expression is strongly associated with Wnt/β-catenin signalling and represents the transporter of gadoxetic acid in hepatocellular carcinoma. J Hepatol. 2014; 61(5):1080–1087. DOI: 10.1016/j.jhep.2014.06.008. PMID: 24946283.
Article42. Torbenson M, McCabe CE, O'Brien DR, Yin J, Bainter T, Tran NH, et al. Morphological heterogeneity in beta-catenin–mutated hepatocellular carcinomas: implications for tumor molecular classification. Hum Pathol. 2022; 119:15–27. DOI: 10.1016/j.humpath.2021.09.009. PMID: 34592239. PMCID: PMC9258524.
Article43. Rhee H, Kim H, Park YN. Clinico-radio-pathological and molecular features of hepatocellular carcinomas with Keratin 19 expression. Liver Cancer. 2020; 9(6):663–681. DOI: 10.1159/000510522. PMID: 33442539. PMCID: PMC7768132.
Article44. Fang JH, Zhou HC, Zhang C, Shang LR, Zhang L, Xu J, et al. A novel vascular pattern promotes metastasis of hepatocellular carcinoma in an epithelial–mesenchymal transition–independent manner. Hepatology. 2015; 62(2):452–465. DOI: 10.1002/hep.27760. PMID: 25711742.
Article45. Hwang SH, Rhee H. Radiologic features of hepatocellular carcinoma related to prognosis. J Liver Cancer. 2023; 23(1):143–156. DOI: 10.17998/jlc.2023.02.16. PMID: 37384030. PMCID: PMC10202237.46. Wood LD, Heaphy CM, Daniel HDJ, Naini BV, Lassman CR, Arroyo MR, et al. Chromophobe hepatocellular carcinoma with abrupt anaplasia: a proposal for a new subtype of hepatocellular carcinoma with unique morphological and molecular features. Mod Pathol. 2013; 26(12):1586–1593. DOI: 10.1038/modpathol.2013.68. PMID: 23640129. PMCID: PMC3974906.
Article47. Torbenson MS. Hepatocellular carcinoma: making sense of morphological heterogeneity, growth patterns, and subtypes. Hum Pathol. 2021; 112:86–101. DOI: 10.1016/j.humpath.2020.12.009. PMID: 33387587. PMCID: PMC9258523.
Article48. Amano H, Itamoto T, Emoto K, Hino H, Asahara T, Shimamoto F. Granulocyte colony-stimulating factor-producing combined hepatocellular/cholangiocellular carcinoma with sarcomatous change. J Gastroenterol. 2005; 40(12):1158–1159. DOI: 10.1007/s00535-005-1715-8. PMID: 16378181.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Pathology and diagnostic approaches to well-differentiated hepatocellular lesions: a narrative review
- Histopathological Variants of Hepatocellular Carcinomas: an Update According to the 5th Edition of the WHO Classification of Digestive System Tumors
- Up-to-date Knowledge on the Pathological Diagnosis of Hepatocellular Carcinoma
- The use of transient elastography for predicting hepatocellular carcinoma in chronic hepatitis B patients: Editorial on “Risk assessment of hepatitis B virus-related hepatocellular carcinoma development using vibration-controlled transient elastography: Systematic review and meta-analysis”
- Genetic insights into sarcomatoid hepatocellular carcinoma: Critical role of ARID2 in pathogenesis and immune feature: Editorial on “Integrated molecular characterization of sarcomatoid hepatocellular carcinoma”