Ann Lab Med.
2024 Nov;44(6):611-613. 10.3343/alm.2024.0234.
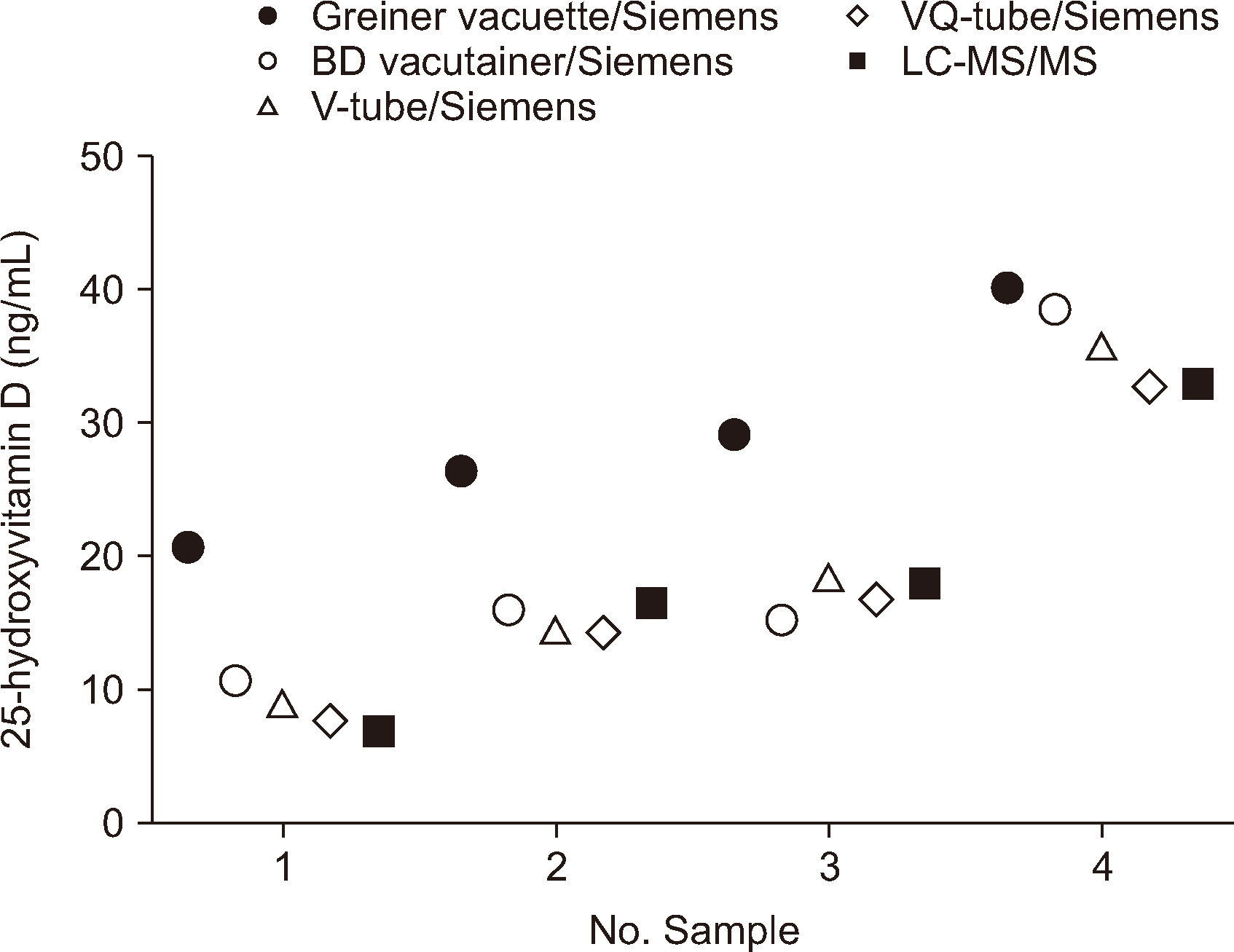
Effect of Blood Collection Tubes on Vitamin D Immunoassay Results
- Affiliations
-
- 1Department of Laboratory Medicine, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 2Department of Endocrine Substance Analysis Center (ESAC), Green Cross Laboratories (GC Labs), Yongin, Korea
- KMID: 2560813
- DOI: http://doi.org/10.3343/alm.2024.0234
Figure
Reference
-
References
1. Bowen RAR, Hortin GL, Csako G, Otaez OH, Remaley AT. 2010; Impact of blood collection devices on clinical chemistry assays. Clin Biochem. 43:4–25. DOI: 10.1016/j.clinbiochem.2009.10.001. PMID: 19822139.2. Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. 2011; Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 96:1911–30. DOI: 10.1210/jc.2011-0385. PMID: 21646368.3. Seo JD, Lee JH, Cho SE, Song J, Lee YW, Yun YM. 2024; Evaluation of test standardization of 25-OH vitamin D immunoassays. Lab Med Online. 14:8–16. DOI: 10.47429/lmo.2024.14.1.8.4. Bowen RAR, Chan Y, Ruddel ME, Hortin GL, Csako G, Demosky SJ Jr., et al. 2005; Immunoassay interference by a commonly used blood collection tube additive, the organosilicone surfactant silwet L-720. Clin Chem. 51:1874–82. DOI: 10.1373/clinchem.2005.055400. PMID: 16099932.5. Yu S, Cheng X, Fang H, Zhang R, Han J, Qin X, et al. 2015; 25OHD analogues and vacuum blood collection tubes dramatically affect the accuracy of automated immunoassays. Sci Rep. 5:14636. DOI: 10.1038/srep14636. PMID: 26420221. PMCID: PMC4588576.6. Yu S, Zhou W, Cheng X, Fang H, Zhang R, Cheng Q, et al. 2016; Blood collection tubes and storage temperature should be evaluated when using the Siemens ADVIA Centaur XP for measuring 25-hydroxyvitamin D. PLoS One. 11:e0166327. DOI: 10.1371/journal.pone.0166327. PMID: 27832181. PMCID: PMC5104342. PMID: 8f4e29c3dba749daa809a3640ed026ad.7. Kricka LJ, Park JY, Senior MB, Fontanilla R. 2005; Processing controls in blood collection tubes reveals interference. Clin Chem. 51:2422–3. DOI: 10.1373/clinchem.2005.060392. PMID: 16306118.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of Two Plastic Vacuum Tubes and Glass Tube for Use in Thyroid Hormone Tests
- The Use of Serum Separation Tube (SST) for Hormone Assay by Chemiluminescence Immunoassay
- Evaluation of EstarVac(R) Evacuated Blood-collection Tubes
- Comparison of Two New Plastic Tubes (Sekisui INSEPACK and Green Cross Green Vac-Tube) with BD Vacutainer Tubes for 49 Analytes
- Comparison of Sekisui Trank Insepack and BD Vacutainer Plastic Citrate Tubes for Routine Coagulation Assays