Ann Lab Med.
2024 Nov;44(6):487-496. 10.3343/alm.2023.0477.
Exploring Appropriate Reference Intervals and Clinical Decision Limits for Glucose-6-Phosphate Dehydrogenase Activity in Individuals From Guangzhou, China
- Affiliations
-
- 1Department of Laboratory Medicine, Zhujiang Hospital, Southern Medical University, Guangzhou, China
- 2Institute of Antibody Engineering, School of Laboratory Medicine and Biotechnology, Southern Medical University, Guangzhou, China
- KMID: 2560795
- DOI: http://doi.org/10.3343/alm.2023.0477
Abstract
- Background
Quantitative detection of glucose-6-phosphate dehydrogenase (G6PD) is commonly done to screen for G6PD deficiency. However, current reference intervals (RIs) of G6PD are unsuitable for evaluating G6PD-activity levels with local populations or associating G6PD variants with hemolysis risk to aid clinical decision-making. We explored appropriate RIs and clinical decision limits (CDLs) for G6PD activity in individuals from Guangzhou, China.
Methods
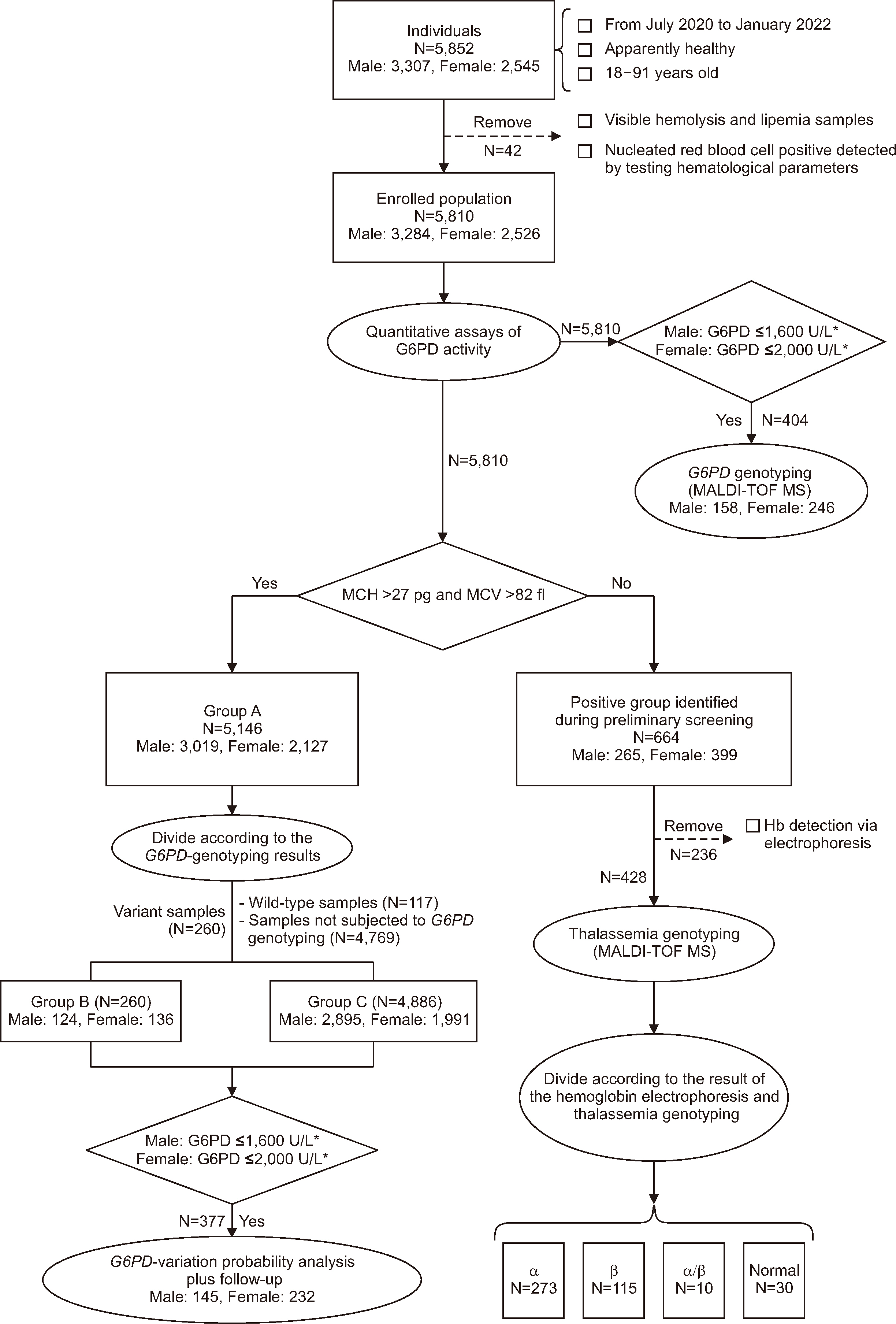
We enrolled 5,852 unrelated individuals between 2020 and 2022 and screened their samples in quantitative assays for G6PD activity. We conducted further investigations, including G6PD genotyping, thalassemia genotyping, follow-up analysis, and statistical analysis, for different groups.
Results
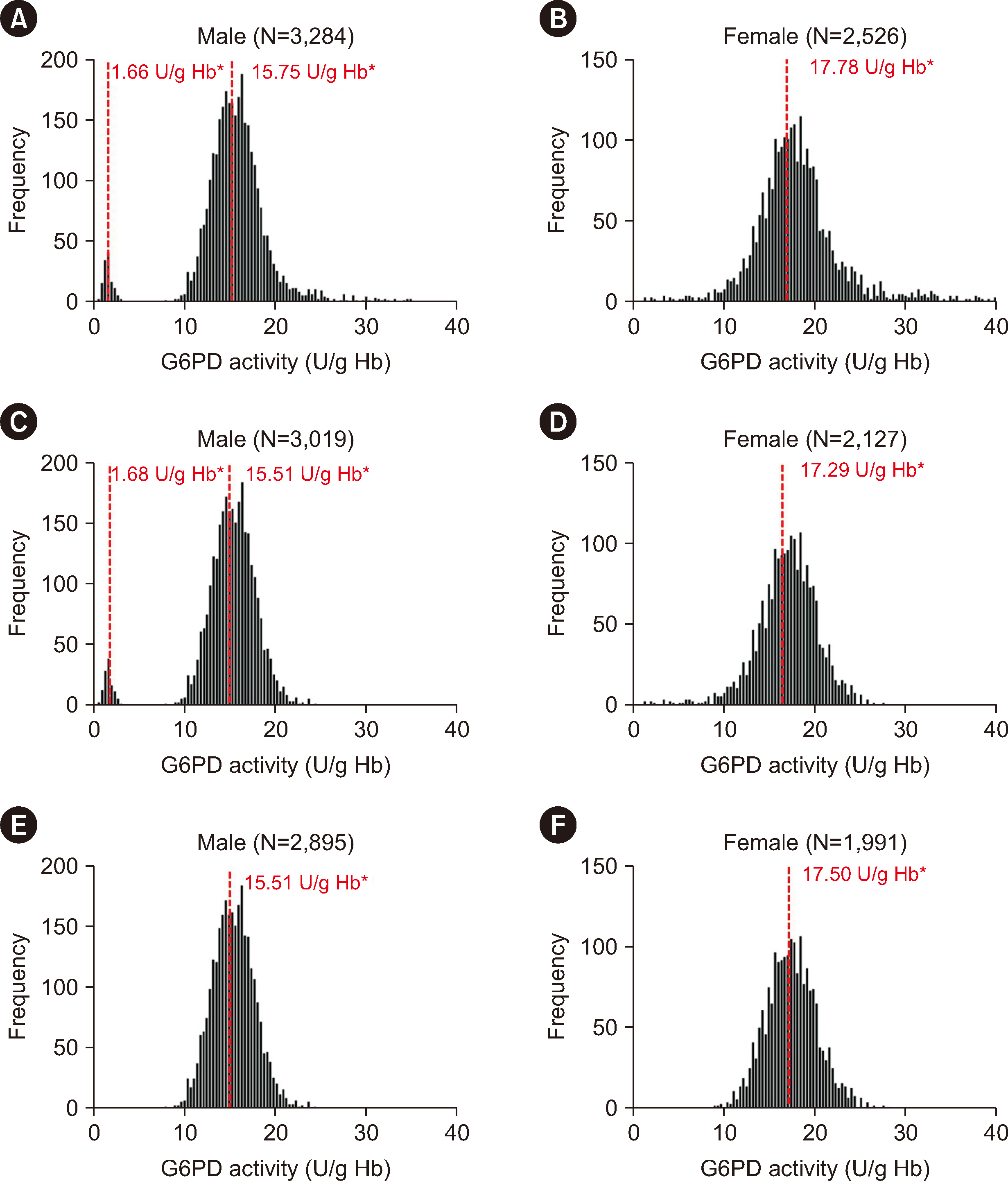
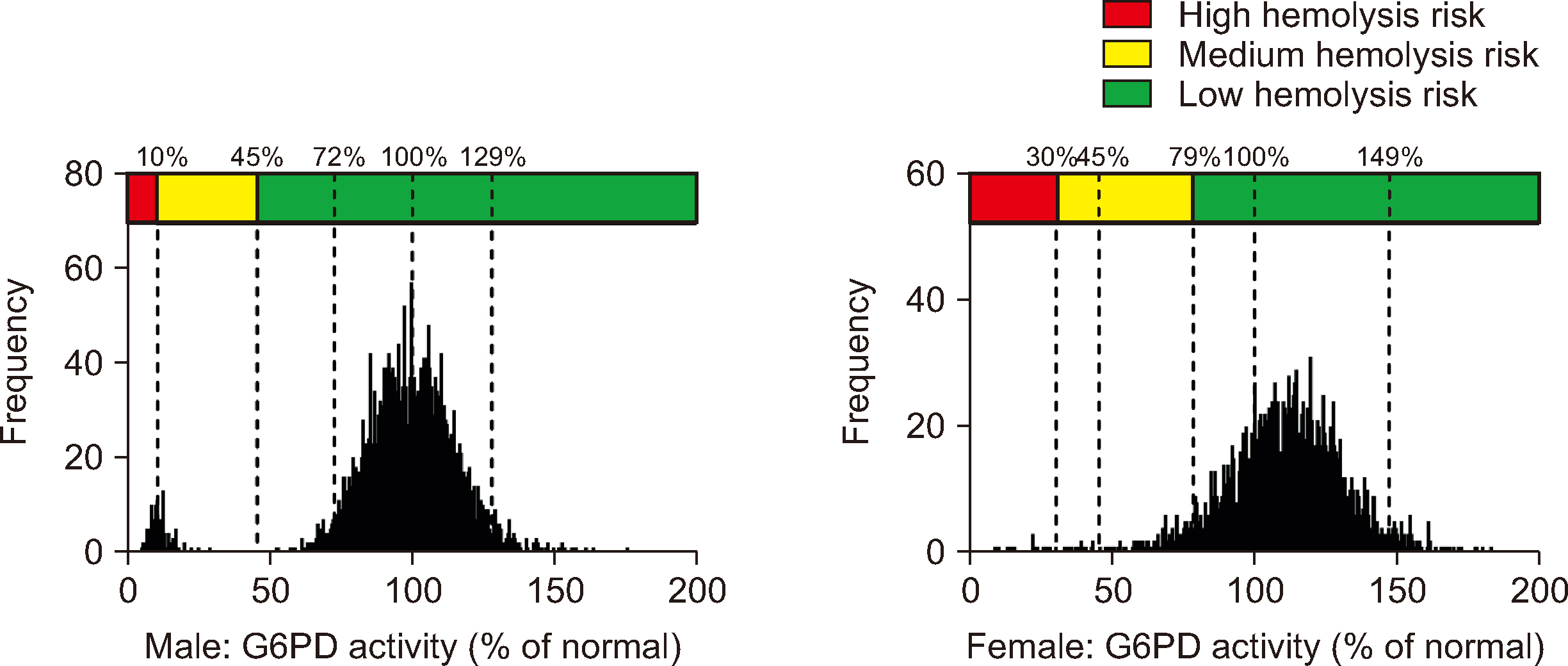
In Guangzhou, the RIs for the G6PD activities were 11.20–20.04 U/g Hb in male and 12.29–23.16 U/g Hb in female. The adjusted male median and normal male median (NMM) values were 15.47 U/g Hb and 15.51 U/g Hb, respectively. A threshold of 45% of the NMM could be used as a CDL to estimate the probability of G6PD variants. Our results revealed high hemolysis-risk CDLs (male: < 10% of the NMM, female: < 30% of the NMM), medium hemolysis-risk CDLs (male: 10%–45% of the NMM, female: 30%–79% of the NMM), and low hemolysis-risk CDLs (male: ≥ 45% of the NMM, female: ≥ 79% of the NMM).
Conclusions
Collectively, our findings contribute to a more accurate evaluation of G6PDactivity levels within the local population and provide valuable insights for clinical decisionmaking. Specifically, identifying threshold values for G6PD variants and hemolysis risk enables improved prediction and management of G6PD deficiency, ultimately enhancing patient care and treatment outcomes.
Keyword
Figure
Reference
-
References
1. Ozarda Y, Sikaris K, Streichert T, Macri J. IFCC Committee on Reference intervals and Decision Limits (C-RIDL). 2018; Distinguishing reference intervals and clinical decision limits - a review by the IFCC Committee on Reference Intervals and Decision Limits. Crit Rev Clin Lab Sci. 55:420–31. DOI: 10.1080/10408363.2018.1482256. PMID: 30047297.2. CLSI. 2010. Defining, establishing, and verifying reference intervals in the clinical laboratory; Approved Guideline. 3rd ed. Clinical and Laboratory Standards Institute;Wayne, PA: CLSI EPC28-A3c.3. Coşkun A, Sandberg S, Unsal I, Cavusoglu C, Serteser M, Kilercik M, et al. 2021; Personalized reference intervals in laboratory medicine: a new model based on within-subject biological variation. Clin Chem. 67:374–84. DOI: 10.1093/clinchem/hvaa233. PMID: 33188412.4. Luzzatto L, Ally M, Notaro R. 2020; Glucose-6-phosphate dehydrogenase deficiency. Blood. 136:1225–40. DOI: 10.1182/blood.2019000944. PMID: 32702756.5. World Health Organization. Technical consultation to review the classification of glucose-6-phosphate dehydrogenase (G6PD). https://www.who.int/publications/m/item/WHO-UCN-GMP-MPAG-2022.01. Updated on Mar 2023.6. Domingo GJ, Satyagraha AW, Anvikar A, Baird K, Bancone G, Bansil P, et al. 2013; G6PD testing in support of treatment and elimination of malaria: recommendations for evaluation of G6PD tests. Malar J. 12:391. DOI: 10.1186/1475-2875-12-391. PMID: 24188096. PMCID: PMC3830439.7. World Health Organization. Guide to G6PD deficiency rapid diagnostic testing to support P. vivax radical cure, 2018. https://www.who.int/publications/i/item/9789241514286. Updated on June 2018.8. Chen Q, Yang X, Huang W, Li Z, Xu M, Li Y, et al. 2023; Target-allele-specific probe single-base extension (TASP-SBE): a novel MALDI-TOF-MS strategy for multi-variants analysis and its application in simultaneous detection of α-/β-thalassemia mutations. Hum Genet. 142:445–56. DOI: 10.1007/s00439-023-02520-w. PMID: 36658365.9. Roper D, Layton M, Rees D, Lambert C, Vulliamy T, De la Salle B, et al. 2020; Laboratory diagnosis of G6PD deficiency. A British Society for Haematology guideline. Br J Haematol. 189:24–38. DOI: 10.1111/bjh.16366. PMID: 31991476.10. World Health Organization. Testing for G6PD deficiency for safe use of primaquine in radical cure of P. vivax and P. ovale. https://www.who.int/publications/i/item/WHO-HTM-GMP-2016.9. Updated on Sep 2016.11. He Y, Zhang Y, Chen X, Wang Q, Ling L, Xu Y. 2020; Glucose-6-phosphate dehydrogenase deficiency in the Han Chinese population: molecular characterization and genotype-phenotype association throughout an activity distribution. Sci Rep. 10:17106. DOI: 10.1038/s41598-020-74200-y. PMID: 33051526. PMCID: PMC7555859.12. Oo NN, Bancone G, Maw LZ, Chowwiwat N, Bansil P, Domingo GJ, et al. 2016; Validation of G6PD point-of-care tests among healthy volunteers in Yangon, Myanmar. PLoS One. 11:e0152304. DOI: 10.1371/journal.pone.0152304. PMID: 27035821. PMCID: PMC4818080. PMID: 82b35d5e7b9a40e691f67427c1401dc2.13. Lai K, Huang G, Su L, He Y. 2017; The prevalence of thalassemia in mainland China: evidence from epidemiological surveys. Sci Rep. 7:920. DOI: 10.1038/s41598-017-00967-2. PMID: 28424478. PMCID: PMC5430438. PMID: 5fe99403708b4a458e51ee0db8191f6a.14. Ye W, Liang Z, Xu Y. 2020; Correlation analysis of G6PD activity level and thalassemia. China Pract Med. 15:95–7.15. Huang S, Zhu W, Chen Q. 2012; Relationship between G6PD activity and thalassemia. Chin J Gen Pract. 10:1140–1.16. Tang L, Feng R. 2021; Correlation analysis between carrying different thalassemia genes and G6PD activity. J Chin Prescrip Drug. 19:159–60.17. Pfeffer DA, Ley B, Howes RE, Adu P, Alam MS, Bansil P, et al. 2020; Correction: quantification of glucose-6-phosphate dehydrogenase activity by spectrophotometry: a systematic review and meta-analysis. PLoS Med. 17:e1003311. DOI: 10.1371/journal.pmed.1003311. PMID: 32706838. PMCID: PMC7380886. PMID: 257666b4af3142bb8fd3d91a5ba44f72.18. Domingo GJ, Advani N, Satyagraha AW, Sibley CH, Rowley E, Kalnoky M, et al. 2019; Addressing the gender-knowledge gap in glucose-6-phosphate dehydrogenase deficiency: challenges and opportunities. Int Health. 11:7–14. DOI: 10.1093/inthealth/ihy060. PMID: 30184203. PMCID: PMC6314154.19. World Health Organization. How to use a G6PD rapid diagnostic test (for detecting glucose-6-phosphate dehydrogenase deficiency): a guide for training at health facility level. https://www.who.int/publications/i/item/WHO-CDS-GMP-2018.15. Updated on Aug 2018.20. Oliveira RAG, Oshiro M, Hirata MH, Hirata RDC, Ribeiro GS, Medeiros TMD, et al. 2009; A novel point mutation in a class IV glucose-6-phosphate dehydrogenase variant (G6PD Sao Paulo) and polymorphic G6PD variants in Sao Paulo State, Brazil. Genet Mol Biol. 32:251–4. DOI: 10.1590/S1415-47572009005000033. PMID: 21637675. PMCID: PMC3036924.21. Lee J, Park J, Choi H, Kim J, Kwon A, Jang W, et al. 2017; Genetic profiles of Korean patients with glucose-6-phosphate dehydrogenase deficiency. Ann Lab Med. 37:108–16. DOI: 10.3343/alm.2017.37.2.108. PMID: 28028996. PMCID: PMC5203987.22. Liu Z, Yu C, Li Q, Cai R, Qu Y, Wang W, et al. 2020; Chinese newborn screening for the incidence of G6PD deficiency and variant of G6PD gene from 2013 to 2017. Hum Mutat. 41:212–21. DOI: 10.1002/humu.23911. PMID: 31489982.23. Lacerda MVG, Llanos-Cuentas A, Krudsood S, Lon C, Saunders DL, Mohammed R, et al. 2019; Single-dose tafenoquine to prevent relapse of Plasmodium vivax malaria. N Engl J Med. 380:215–28. DOI: 10.1056/NEJMoa1710775. PMID: 30650322. PMCID: PMC6657226.24. Commons RJ, McCarthy JS, Price RN. 2020; Tafenoquine for the radical cure and prevention of malaria: the importance of testing for G6PD deficiency. Med J Aust. 212:152–153.e1. DOI: 10.5694/mja2.50474. PMID: 32036613. PMCID: PMC7064913.25. Li Z, Huang Z, Liu Y, Cao Y, Li Y, Fang Y, et al. 2023; Genotypic and phenotypic characterization of glucose-6-phosphate dehydrogenase (G6PD) deficiency in Guangzhou, China. Hum Genomics. 17:26. DOI: 10.1186/s40246-023-00473-9. PMID: 36949502. PMCID: PMC10035184. PMID: 7b247618716b450f8dd08c2481028acf.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Jaundice and Hemolytic Anemia Appearing within the First 24 Hour of Life due to Glucose-6-Phosphate Dehydrogenase Deficiency
- Enhancement of Plasmacytoma Cell Growth by Ascorbic Acid is Mediated Via Glucose 6-phosphate Dehydrogenase
- Effect of dietary zinc deficiency on the enzymatic components of free radical defense system in the skin of rats
- Functional Reference Limits: Describing Physiological Relationships and Determination of Physiological Limits for Enhanced Interpretation of Laboratory Results
- A Case of Glucose-6-phosphate dehydrogenase Riley Causing Hemolytic Anemia