Korean J Physiol Pharmacol.
2024 Nov;28(6):549-558. 10.4196/kjpp.2024.28.6.549.
Salidroside attenuates sepsis-induced acute lung injury by inhibiting ferroptosis-dependent pathway
- Affiliations
-
- 1Intensive Care Unit, Lanzhou University Second Hospital, Lanzhou University, Lanzhou 730030, Gansu, China
- 2The Second Clinical Medical School, Lanzhou University, Lanzhou 730030, Gansu, China
- 3Emergency Center, Lanzhou University Second Hospital, Lanzhou University, Lanzhou 730030, Gansu, China
- KMID: 2560734
- DOI: http://doi.org/10.4196/kjpp.2024.28.6.549
Abstract
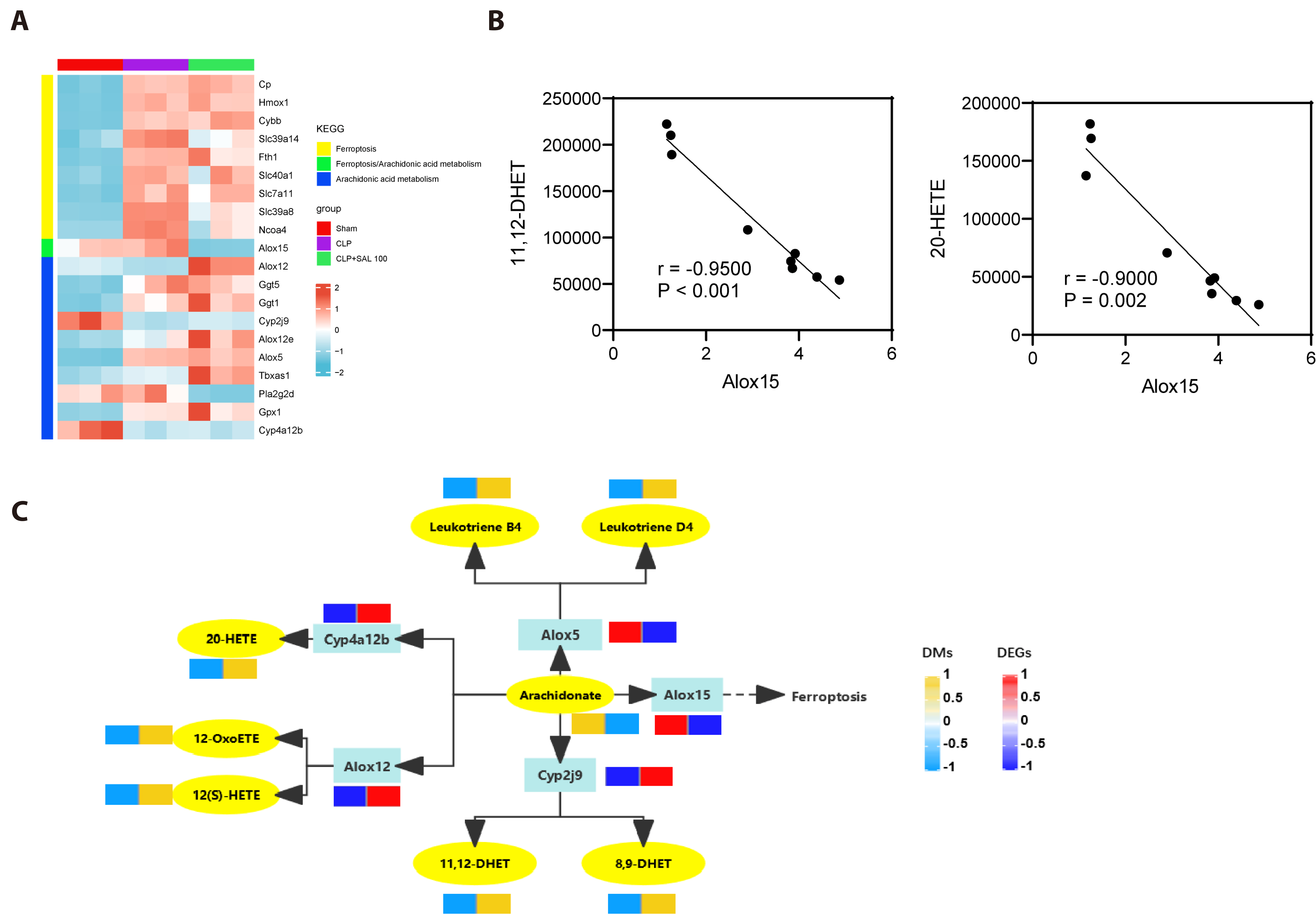
- Sepsis triggers a systemic inflammatory response that can lead to acute lung injury (ALI). Salidroside (SAL) has many pharmacological activities such as antiinflammatory and anti-oxidation. The objective of the study was to explore the mechanism of SAL on ALI caused by sepsis. A model of ALI in septic mice was established by cecal ligation and puncture. Following SAL treatment, the effect of SAL on the ferroptosis pathway in mice was analyzed. The pathological damage of lung tissue, the levels of inflammatory factors and apoptosis in bronchoalveolar lavage fluid (BALF) of mice were evaluated, and the changes of gene expression level and metabolite content abundance were explored by combining transcriptomics and metabolomics analysis. The effect of SAL on ferroptosis in mice with lung injury was observed by intraperitoneal injection of ferroptosis activator Erastin or ferroptosis inhibitor Ferrostatin-1 to promote or inhibit ferroptosis in mice. SAL significantly alleviated the pathological damage of lung tissue, decreased the number of TUNEL positive cells and the levels of TNF-α, IL-1β, IL-6 in BALF, and increased the level of IL-10 in lung injury mice. Moreover, the Fe 2+ content and malondialdehyde decreased significantly, the reactive oxygen species and glutathione content increased significantly, and the arachidonic acid metabolites 20-hydroxyeicosatetraenoic acid (20-HETE), (5Z, 8Z, 10E, 14Z)-12-Oxoeicosa-5,8,10,14-tetraenoic acid (12-OxOETE), (5Z, 8Z, 10E, 14Z)-(12S)-12-Hydroxyeicosa-5,8,10,14-tetraenoic acid (12(S)-HETE), (5Z, 8Z, 14Z)-11,12-Dihydroxyeicosa-5,8,14-trienoic acid (11,12-DHET), (5Z, 11Z, 14Z)-8,9-Dihydroxyeicosa-5,11,14-trienoic acid, Leukotriene B4, Leukotriene D4 were significantly up-regulated after SAL treatment. Salidroside alleviates ALI caused by sepsis by inhibiting ferroptosis.
Figure
Reference
-
1. Jiang W, Ma C, Bai J, Du X. 2022; Macrophage SAMSN1 protects against sepsis-induced acute lung injury in mice. Redox Biol. 56:102432. Erratum in: Redox Biol. 2024;70:103036. DOI: 10.1016/j.redox.2024.103036. PMID: 35981417. PMCID: PMC9418554.2. Hwang JS, Kim KH, Park J, Kim SM, Cho H, Lee Y, Han IO. 2019; Glucosamine improves survival in a mouse model of sepsis and attenuates sepsis-induced lung injury and inflammation. J Biol Chem. 294:608–622. DOI: 10.1074/jbc.RA118.004638. PMID: 30455348. PMCID: PMC6333887.3. Zhang J, Zheng Y, Wang Y, Wang J, Sang A, Song X, Li X. 2022; YAP1 alleviates sepsis-induced acute lung injury via inhibiting ferritinophagy-mediated ferroptosis. Front Immunol. 13:884362. DOI: 10.3389/fimmu.2022.884362. PMID: 35979359. PMCID: PMC9376389.4. Ingold I, Berndt C, Schmitt S, Doll S, Poschmann G, Buday K, Roveri A, Peng X, Porto Freitas F, Seibt T, Mehr L, Aichler M, Walch A, Lamp D, Jastroch M, Miyamoto S, Wurst W, Ursini F, Arnér ESJ, Fradejas-Villar N, et al. 2018; Selenium utilization by GPX4 is required to prevent hydroperoxide-induced ferroptosis. Cell. 172:409–422.e21. DOI: 10.1016/j.cell.2017.11.048. PMID: 29290465.5. Alim I, Caulfield JT, Chen Y, Swarup V, Geschwind DH, Ivanova E, Seravalli J, Ai Y, Sansing LH, Ste Marie EJ, Hondal RJ, Mukherjee S, Cave JW, Sagdullaev BT, Karuppagounder SS, Ratan RR. 2019; Selenium drives a transcriptional adaptive program to block ferroptosis and treat stroke. Cell. 177:1262–1279.e25. DOI: 10.1016/j.cell.2019.03.032. PMID: 31056284.6. Kim MJ, Yun GJ, Kim SE. 2021; Metabolic regulation of ferroptosis in cancer. Biology (Basel). 10:83. DOI: 10.3390/biology10020083. PMID: 33499222. PMCID: PMC7911352.7. Molchanova AY, Rjabceva SN, Melik-Kasumov TB, Pestov NB, Angelova PR, Shmanai VV, Sharko OL, Bekish AV, James G, Park HG, Udalova IA, Brenna JT, Shchepinov MS. 2022; Deuterated arachidonic acid ameliorates lipopolysaccharide-induced lung damage in mice. Antioxidants (Basel). 11:681. DOI: 10.3390/antiox11040681. PMID: 35453366. PMCID: PMC9027010.8. Xu F, Xu J, Xiong X, Deng Y. 2019; Salidroside inhibits MAPK, NF-κB, and STAT3 pathways in psoriasis-associated oxidative stress via SIRT1 activation. Redox Rep. 24:70–74. DOI: 10.1080/13510002.2019.1658377. PMID: 31495284. PMCID: PMC6748574.9. Zhong Z, Han J, Zhang J, Xiao Q, Hu J, Chen L. 2018; Pharmacological activities, mechanisms of action, and safety of salidroside in the central nervous system. Drug Des Devel Ther. 12:1479–1489. DOI: 10.2147/DDDT.S160776. PMID: 29872270. PMCID: PMC5973445.10. Huang X, Xue H, Ma J, Zhang Y, Zhang J, Liu Y, Qin X, Sun C. 2019; Salidroside ameliorates Adriamycin nephropathy in mice by inhibiting β-catenin activity. J Cell Mol Med. 23:4443–4453. DOI: 10.1111/jcmm.14340. PMID: 30993911. PMCID: PMC6533469.11. Hasin Y, Seldin M, Lusis A. 2017; Multi-omics approaches to disease. Genome Biol. 18:83. DOI: 10.1186/s13059-017-1215-1. PMID: 28476144. PMCID: PMC5418815.12. Xie X, Liao J, Ai Y, Gao J, Zhao J, Qu F, Xu C, Zhang Z, Wen W, Cui H, Wang H. 2021; Pi-Dan-Jian-Qing decoction ameliorates type 2 diabetes mellitus through regulating the gut microbiota and serum metabolism. Front Cell Infect Microbiol. 11:748872. DOI: 10.3389/fcimb.2021.748872. PMID: 34938667. PMCID: PMC8685325.13. Bylesjö M, Rantalainen M, Cloarec O, Nicholson JK, Holmes E, Trygg J. 2006; OPLS discriminant analysis: combining the strengths of PLS-DA and SIMCA classification. J Chemom. 20:341–351. DOI: 10.1002/cem.1006.14. Tang H, Gao L, Mao J, He H, Liu J, Cai X, Lin H, Wu T. 2016; Salidroside protects against bleomycin-induced pulmonary fibrosis: activation of Nrf2-antioxidant signaling, and inhibition of NF-κB and TGF-β1/Smad-2/-3 pathways. Cell Stress Chaperones. 21:239–249. DOI: 10.1007/s12192-015-0654-4. PMID: 26577463. PMCID: PMC4786523.15. Liu MW, Su MX, Qin LF, Liu X, Tian ML, Zhang W, Wang YH. 2014; Effect of salidroside on lung injury by upregulating peroxisome proliferator-activated receptor γ expression in septic rats. Exp Ther Med. 7:1446–1456. DOI: 10.3892/etm.2014.1629. PMID: 24926325. PMCID: PMC4043580.16. Wang Y, Xu CF, Liu YJ, Mao YF, Lv Z, Li SY, Zhu XY, Jiang L. 2017; Salidroside attenuates ventilation induced lung injury via SIRT1-dependent inhibition of NLRP3 inflammasome. Cell Physiol Biochem. 42:34–43. DOI: 10.1159/000477112. PMID: 28490015.17. Fan H, Su BJ, Le JW, Zhu JH. 2022; Salidroside protects acute kidney injury in septic rats by inhibiting inflammation and apoptosis. Drug Des Devel Ther. 16:899–907. DOI: 10.2147/DDDT.S361972. PMID: 35386851. PMCID: PMC8978577.18. Jiang L, Xu L, Zheng L, Wang Y, Zhuang M, Yang D. 2022; Salidroside attenuates sepsis-associated acute lung injury through PPP1R15A mediated endoplasmic reticulum stress inhibition. Bioorg Med Chem. 71:116865. DOI: 10.1016/j.bmc.2022.116865. PMID: 35985062.19. Wang Y, Chen Z, Luo J, Zhang J, Sang AM, Cheng ZS, Li XY. 2023; Salidroside postconditioning attenuates ferroptosis-mediated lung ischemia-reperfusion injury by activating the Nrf2/SLC7A11 signaling axis. Int Immunopharmacol. 115:109731. Erratum in: Int Immunopharmacol. DOI: 10.1016/j.intimp.2023.110002. PMID: 36907990.20. Chen X, Li J, Kang R, Klionsky DJ, Tang D. 2021; Ferroptosis: machinery and regulation. Autophagy. 17:2054–2081. DOI: 10.1080/15548627.2020.1810918. PMID: 32804006. PMCID: PMC8496712.21. Kagan VE, Mao G, Qu F, Angeli JP, Doll S, Croix CS, Dar HH, Liu B, Tyurin VA, Ritov VB, Kapralov AA, Amoscato AA, Jiang J, Anthonymuthu T, Mohammadyani D, Yang Q, Proneth B, Klein-Seetharaman J, Watkins S, Bahar I, et al. 2017; Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat Chem Biol. 13:81–90. DOI: 10.1038/nchembio.2238. PMID: 27842066. PMCID: PMC5506843.22. Tang C, Tang Y, Wang Q, Chu D, Zhou J, Zhou Y. 2022; Yangyinqingfei decoction attenuates PM2.5-induced lung injury by enhancing arachidonic acid metabolism. Front Pharmacol. 13:1056078. DOI: 10.3389/fphar.2022.1056078. PMID: 36467030. PMCID: PMC9708729.23. Lewis RA, Austen KF, Soberman RJ. 1990; Leukotrienes and other products of the 5-lipoxygenase pathway. Biochemistry and relation to pathobiology in human diseases. N Engl J Med. 323:645–655. DOI: 10.1056/NEJM199009063231006. PMID: 2166915.24. Lee JY, Nam M, Son HY, Hyun K, Jang SY, Kim JW, Kim MW, Jung Y, Jang E, Yoon SJ, Kim J, Kim J, Seo J, Min JK, Oh KJ, Han BS, Kim WK, Bae KH, Song J, Kim J, et al. 2020; Polyunsaturated fatty acid biosynthesis pathway determines ferroptosis sensitivity in gastric cancer. Proc Natl Acad Sci U S A. 117:32433–32442. DOI: 10.1073/pnas.2006828117. PMID: 33288688. PMCID: PMC7768719.25. Sun Y, Chen P, Zhai B, Zhang M, Xiang Y, Fang J, Xu S, Gao Y, Chen X, Sui X, Li G. 2020; The emerging role of ferroptosis in inflammation. Biomed Pharmacother. 127:110108. DOI: 10.1016/j.biopha.2020.110108. PMID: 32234642.26. Gao M, Deng J, Liu F, Fan A, Wang Y, Wu H, Ding D, Kong D, Wang Z, Peer D, Zhao Y. 2019; Triggered ferroptotic polymer micelles for reversing multidrug resistance to chemotherapy. Biomaterials. 223:119486. DOI: 10.1016/j.biomaterials.2019.119486. PMID: 31520887.27. Reichert CO, de Freitas FA, Sampaio-Silva J, Rokita-Rosa L, Barros PL, Levy D, Bydlowski SP. 2020; Ferroptosis mechanisms involved in neurodegenerative diseases. Int J Mol Sci. 21:8765. DOI: 10.3390/ijms21228765. PMID: 33233496. PMCID: PMC7699575.28. Zhao J, Piao X, Wu Y, Liang S, Han F, Liang Q, Shao S, Zhao D. 2020; Cepharanthine attenuates cerebral ischemia/reperfusion injury by reducing NLRP3 inflammasome-induced inflammation and oxidative stress via inhibiting 12/15-LOX signaling. Biomed Pharmacother. 127:110151. DOI: 10.1016/j.biopha.2020.110151. PMID: 32559840.29. Łuczaj W, Gęgotek A, Skrzydlewska E. 2017; Antioxidants and HNE in redox homeostasis. Free Radic Biol Med. 111:87–101. DOI: 10.1016/j.freeradbiomed.2016.11.033. PMID: 27888001.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Ferroptosis Is Crucial for Cisplatin Induced Sertoli Cell Injury via N6-Methyladenosine Dependent Manner
- Oligomeric proanthocyanidin ameliorates sepsis-associated renal tubular injury: involvement of oxidative stress, inflammation, PI3K/AKT and NFκκB signaling pathways
- Effects of Gabexate Mesilate (Foy(R)) on Endotoxin-Induced Acute Lung Injury in Rabbit
- Rbbp6-Mediated Bmal1 Ubiquitination Inhibits YAP1 Signaling Pathway to Promote Ferroptosis in Diabetes-Induced Testicular Damage
- Effects of Propofol and Gabexate Mesilate (Foy) on Endotoxin-induced Acute Lung Injury in Rabbit