Korean J Gastroenterol.
2024 Oct;84(4):177-187. 10.4166/kjg.2024.094.
Acute Gastropathy Associated with Bowel Preparation According to Age: Oral Sulfate Tablets versus 1-L Polyethylene Glycol with Ascorbic Acid
- Affiliations
-
- 1Department of Internal Medicine, Kyung Hee University Hospital at Gang Dong, College of Medicine, Kyung Hee University, Seoul, Korea
- KMID: 2560709
- DOI: http://doi.org/10.4166/kjg.2024.094
Abstract
- Background/Aims
The use of 1-L polyethylene glycol with ascorbate (PEG/Asc) and oral sulfate tablets (OST) as low-volume bowel preparation agents has gradually increased. However, these agents may induce acute gastropathy during bowel preparation, particularly in elderly populations. This study aimed to compare the incidence of acute gastropathy of 1-L PEG/Asc and OST according to age, as well as efficacy and safety.
Methods
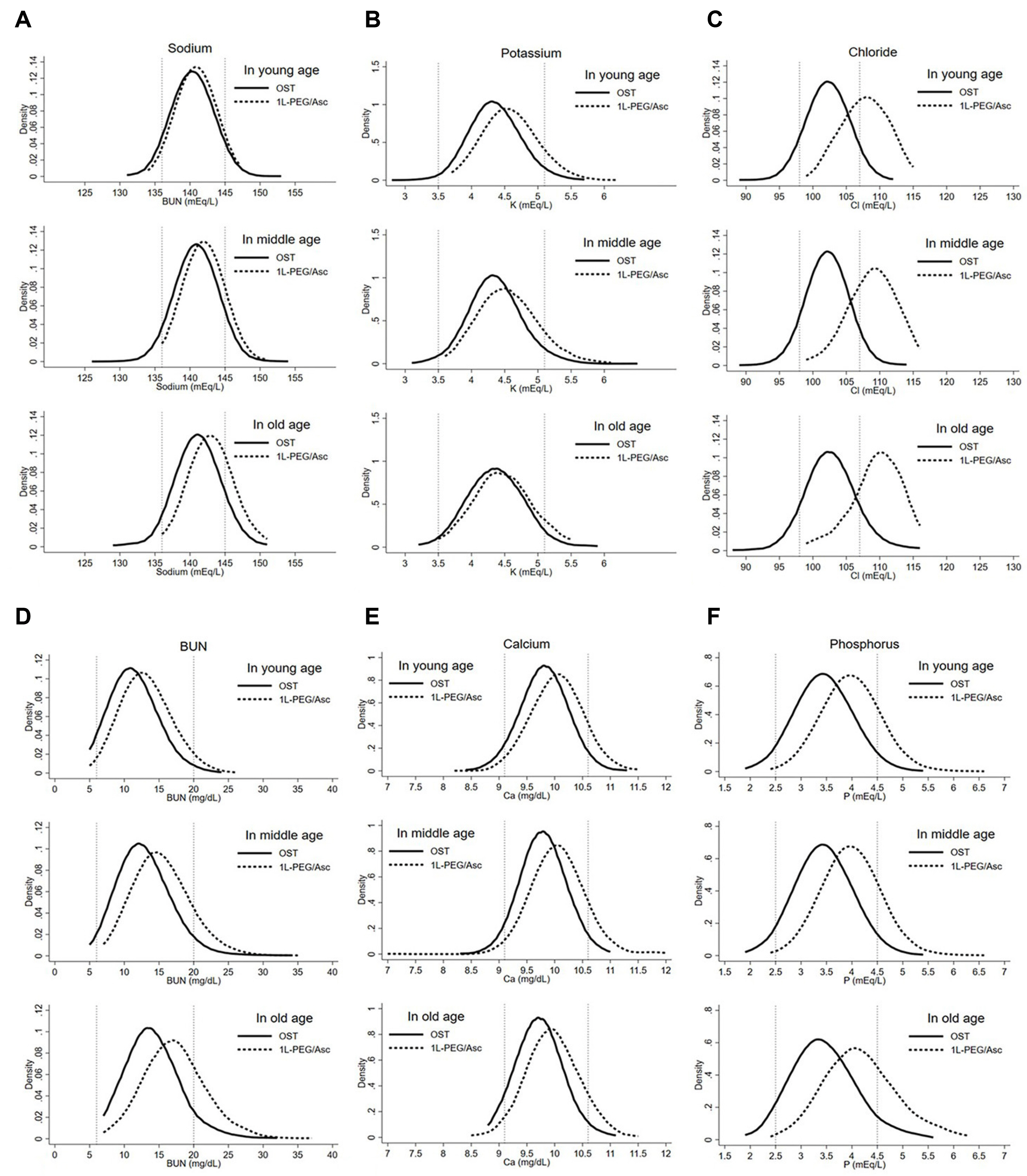
This retrospective study included patients who underwent esophagogastroduodenoscopy (EGD) and colonoscopy for screening on the same day and underwent bowel preparation using OST or 1-L PEG/Asc. We collected EGD findings related to acute gastropathy, bowel-cleansing score using the Boston Bowel Preparation Scale (BBPS), polyp or adenoma detection rate (ADR), and laboratory parameters.
Results
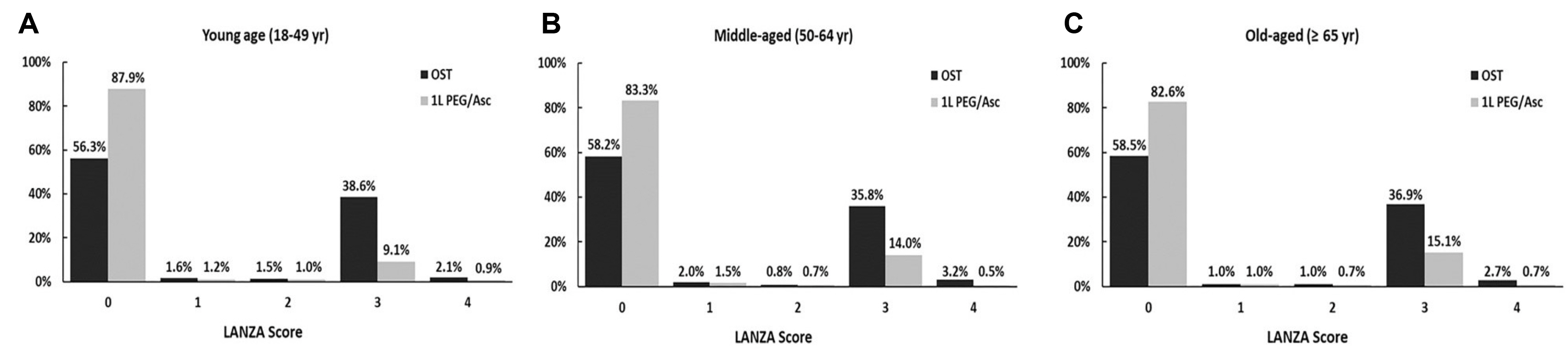
Of 4,711 patients, 1,758, 2,241, and 712 were in the younger (18–49 years), middle-aged (50–64 years), and older (≥65 years) groups, respectively. In all age groups, the OST group had higher rates of acute gastropathy than the 1-L PEG/Asc group. The younger-, middle-, and older-aged groups had OST and 1-L PEG/Asc usage rates of 42.9% and 11.6%, 41.2% and 16.0%, and 41.5% and 16.4%, respectively. Notably, in the younger group, the total BBPS and ADR scores were significantly higher in the OST group than in the 1-L PEG/Asc group; however, these did not differ in the other age groups.
Conclusions
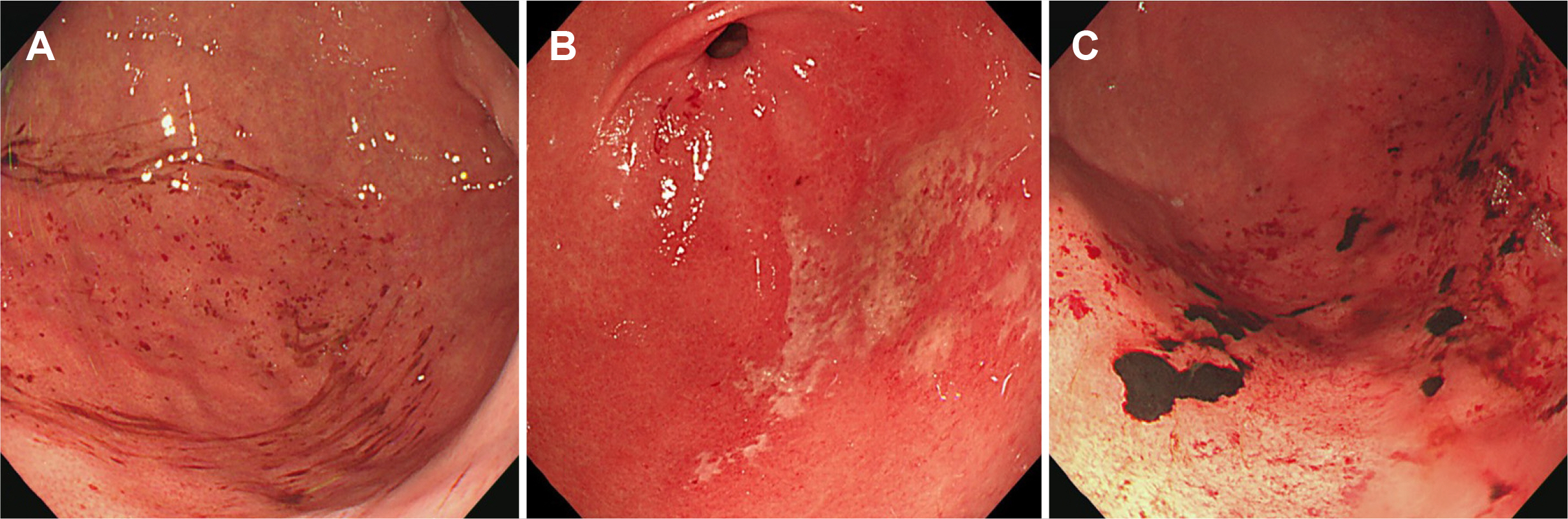
Acute gastropathy was more strongly associated with OST than with 1-L PEG/Asc in all age groups. Therefore, physicians should consider acute gastropathy associated with low-volume agents in all age groups when performing bowel preparation.
Figure
Reference
-
1. Nishihara R, Wu K, Lochhead P, et al. 2013; Long-term colorectal-cancer incidence and mortality after lower endoscopy. N Engl J Med. 369:1095–1105. DOI: 10.1056/NEJMoa1301969. PMID: 24047059. PMCID: PMC3840160.2. Løberg M, Kalager M, Holme Ø, Hoff G, Adami HO, Bretthauer M. 2014; Long-term colorectal-cancer mortality after adenoma removal. N Engl J Med. 371:799–807. DOI: 10.1056/NEJMoa1315870. PMID: 25409381.3. Kaminski MF, Regula J, Kraszewska E, et al. 2010; Quality indicators for colonoscopy and the risk of interval cancer. N Engl J Med. 362:1795–1803. DOI: 10.1056/NEJMoa0907667. PMID: 20463339.4. DeMicco MP, Clayton LB, Pilot J, Epstein MS. NOCT Study Group. 2018; Novel 1 L polyethylene glycol-based bowel preparation NER1006 for overall and right-sided colon cleansing: a randomized controlled phase 3 trial versus trisulfate. Gastrointest Endosc. 87:677–687.e3. DOI: 10.1016/j.gie.2017.07.047. PMID: 28803744.5. Lee HH, Lim CH, Kim JS, et al. 2019; Comparison between an oral sulfate solution and a 2 L of Polyethylene glycol/ascorbic acid as a split dose bowel preparation for colonoscopy. J Clin Gastroenterol. 53:e431–e437. DOI: 10.1097/MCG.0000000000001137. PMID: 30308546.6. Repici A, Coron E, Sharma P, et al. 2019; Improved high-quality colon cleansing with 1L NER1006 versus 2L polyethylene glycol + ascorbate or oral sulfate solution. Dig Liver Dis. 51:1671–1677. DOI: 10.1016/j.dld.2019.06.026. PMID: 31409579.7. Woo JH, Koo HS, Kim DS, Shin JE, Jung Y, Huh KC. 2022; Evaluation of the efficacy of 1 L polyethylene glycol plus ascorbic acid and an oral sodium sulfate solution: A multi-center, prospective randomized controlled trial. Medicine (Baltimore). 101:e30355. DOI: 10.1097/MD.0000000000030355. PMID: 36107563. PMCID: PMC9439845.8. Di Palma JA, Bhandari R, Cleveland MV, et al. 2021; A safety and efficacy comparison of a new sulfate-based tablet bowel preparation versus a PEG and ascorbate comparator in adult subjects undergoing colonoscopy. Am J Gastroenterol. 116:319–328. DOI: 10.14309/ajg.0000000000001020. PMID: 33165006. PMCID: PMC7864663.9. Park JH, Hong SW, Hwang SW, et al. 2023; Efficacy and safety of oral sodium sulfate tablet compared with 1-L polyethylene glycol plus ascorbate: a prospective, randomized, endoscopist-blinded trial. J Gastroenterol Hepatol. 38:2090–2096. DOI: 10.1111/jgh.16343. PMID: 37655723.10. Kim JH, Park YE, Kim TO, et al. 2022; Comparison of the efficacy and safety between oral sulfate tablet and polyethylene glycol for bowel preparation before colonoscopy according to age. Medicine (Baltimore). 101:e29884. DOI: 10.1097/MD.0000000000029884. PMID: 35801801. PMCID: PMC9259131.11. Lee SE, Oh DJ, Nam JH, et al. 2023; Taking oral sulfate tablets with simethicone for bowel preparation leads to higher adenoma detection rate than polyethylene glycol: A propensity score analysis. Dig Dis Sci. 68:867–876. DOI: 10.1007/s10620-022-07611-8. PMID: 35781655.12. Song JH, Bae JH, Yim JY. 2023; Efficacy of oral sulfate tablets for bowel preparation and adenoma detection rate. J Gastroenterol Hepatol. 38:410–415. DOI: 10.1111/jgh.16079. PMID: 36453642.13. Nam SY, Choi IJ, Park KW, et al. 2010; Risk of hemorrhagic gastropathy associated with colonoscopy bowel preparation using oral sodium phosphate solution. Endoscopy. 42:109–113. DOI: 10.1055/s-0029-1243797. PMID: 20140827.14. Suh JP, Choi YS, Lee SH. 2014; Education and Imaging. Gastroenterology: acute mucosal injury of esophagus and stomach induced by sodium picosulfate/magnesium citrate for bowel preparation. J Gastroenterol Hepatol. 29:1571. DOI: 10.1111/jgh.12633. PMID: 25073632.15. Ze EY, Choi CH, Kim JW. 2017; Acute gastric injury caused by undissolved sodium picosulfate/magnesium citrate powder. Clin Endosc. 50:87–90. DOI: 10.5946/ce.2016.081. PMID: 27732774. PMCID: PMC5299977.16. Seo JY, Kang KJ, Kang HS, et al. 2015; Corrosive esophagitis caused by ingestion of picosulfate. Clin Endosc. 48:66–69. DOI: 10.5946/ce.2015.48.1.66. PMID: 25674529. PMCID: PMC4323436.17. Villa E, Bansal M, Zakko W. S578 Oral sulfate tablet bowel preparation is associated with erosive gastritis. Am J Gastroenerol. 2021; 116:S263. DOI: 10.14309/01.ajg.0000774788.09911.e3.18. Yoon JY, Kim HG, Cho YS, Kim HI, Cha JM. 2022; 1 L- versus 2 L-polyethylene glycol with ascorbic acid for bowel preparation in elderly patients: a randomized multicenter study. Surg Endosc. 36:5724–5733. DOI: 10.1007/s00464-021-08947-4. PMID: 35031868.19. Cohen LB, Sanyal SM, Von Althann C, et al. 2010; Clinical trial: 2-L polyethylene glycol-based lavage solutions for colonoscopy preparation- a randomized, single-blind study of two formulations. Aliment Pharmacol Ther. 32:637–644. DOI: 10.1111/j.1365-2036.2010.04390.x. PMID: 20626383.20. Cha JM, Kozarek RA, La Selva D, et al. 2016; Risks and benefits of colonoscopy in patients 90 years or older, compared with younger patients. Clin Gastroenterol Hepatol. 14:80–86.e1. DOI: 10.1016/j.cgh.2015.06.036. PMID: 26164224.21. McGettigan MJ, Menias CO, Gao ZJ, Mellnick VM, Hara AK. 2016; Imaging of drug-induced complications in the gastrointestinal system. Radiographics. 36:71–87. DOI: 10.1148/rg.2016150132. PMID: 26761532.22. Lanza FL, Collaku A, Liu DJ. 2018; Endoscopic comparison of gastroduodenal injury with over-the-counter doses of new fast-dissolving ibuprofen and paracetamol formulations: a randomized, placebo-controlled, 4-way crossover clinical trial. Clin Exp Gastroenterol. 11:169–177. DOI: 10.2147/CEG.S153231. PMID: 29713191. PMCID: PMC5907787.23. Calderwood AH, Schroy PC 3rd, Lieberman DA, Logan JR, Zurfluh M, Jacobson BC. 2014; Boston Bowel Preparation Scale scores provide a standardized definition of adequate for describing bowel cleanliness. Gastrointest Endosc. 80:269–276. DOI: 10.1016/j.gie.2014.01.031. PMID: 24629422. PMCID: PMC4104141.24. Hawkey CJ. 2000; Nonsteroidal anti-inflammatory drug gastropathy. Gastroenterology. 119:521–535. DOI: 10.1053/gast.2000.9561. PMID: 10930388.25. Coron E, Dewitte M, Aubert P, Musquer N, Neunlist M, Bruley des Varannes S. 2015; Reversibility of gastric mucosal lesions induced by sodium phosphate tablets and characterized by probe-based confocal laser endomicroscopy. Endosc Int Open. 3:E69–E75. DOI: 10.1055/s-0034-1377934. PMID: 26134776. PMCID: PMC4423282.26. Matsukuma K, Gui D, Olson KA, Tejaswi S, Clayton EF, Thai A. 2016; OsmoPrep-associated gastritis: A histopathologic mimic of iron pill gastritis and mucosal calcinosis. Am J Surg Pathol. 40:1550–1556. DOI: 10.1097/PAS.0000000000000706. PMID: 27454942. PMCID: PMC5156324.27. Park YE, Jeong SJ, Lee J, et al. 2022; Multi-center study of residual gastric volume and bowel preparation after the usage of 1L and 2L polyethylene glycol in Korea. Medicine (Baltimore). 101:e30795. DOI: 10.1097/MD.0000000000030795. PMID: 36197218. PMCID: PMC9509098.28. Di Palma JA, Rodriguez R, McGowan J, Cleveland Mv. 2009; A randomized clinical study evaluating the safety and efficacy of a new, reduced-volume, oral sulfate colon-cleansing preparation for colonoscopy. Am J Gastroenterol. 104:2275–2284. DOI: 10.1038/ajg.2009.389. PMID: 19584830.29. Jeon SR, Park SK, Yang DH, Cha JM. 2023; Comparison of a novel mini-oral sulfate tablet and the conventional oral sulfate tablet in bowel preparation for colonoscopy: a prospective, randomized, investigator-blinded, multicenter, non-inferior, phase 3 trial. J Gastroenterol. 58:1114–1123. DOI: 10.1007/s00535-023-02023-5. PMID: 37542674.30. Rex DK. 2019; Hyperosmotic low-volume bowel preparations: Is NER1006 safe? Gastrointest Endosc. 89:656–658. DOI: 10.1016/j.gie.2018.11.009. PMID: 30448411.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Optimal and Safe Bowel Preparation for Colonoscopy
- How to Choose the Optimal Bowel Preparation Regimen for Colonoscopy
- Acute Gastropathy Associated with Bowel Preparation for Colonoscopy
- Comparison of Oral Sulfate Solution and Polyethylene Glycol Plus Ascorbic Acid on the Efficacy of Bowel Preparation
- Split-dose Bowel Preparation for Colonoscopy: 2 Liters Polyethylene Glycol with Ascorbic Acid versus Sodium Picosulfate versus Oral Sodium Phosphate Tablets