Korean J Gastroenterol.
2024 Aug;84(2):82-89. 10.4166/kjg.2024.068.
Acute Gastropathy Associated with Bowel Preparation for Colonoscopy
- Affiliations
-
- 1Department of Internal Medicine, Kyung Hee University Hospital at Gang Dong, College of Medicine, Kyung Hee University, Seoul, Korea
- KMID: 2558899
- DOI: http://doi.org/10.4166/kjg.2024.068
Abstract
- Background/Aims
Utilization of low-volume preparation agents is crucial to improve patient willingness to undergo repeat colonoscopies. However, gastric safety data on preparation agents are limited. This study evaluated the acute gastropathy associated with bowel preparation agents.
Methods
This retrospective study enrolled healthy subjects who underwent both esophagogastroduodenoscopy and colonoscopy screening. Baseline patient characteristics, bowel preparation success, acute gastropathy, and polyp and adenoma detection rates were evaluated for 1 L polyethylene glycol with ascorbic acid (1 L PEG/Asc) and oral sulfate tablet (OST) groups.
Results
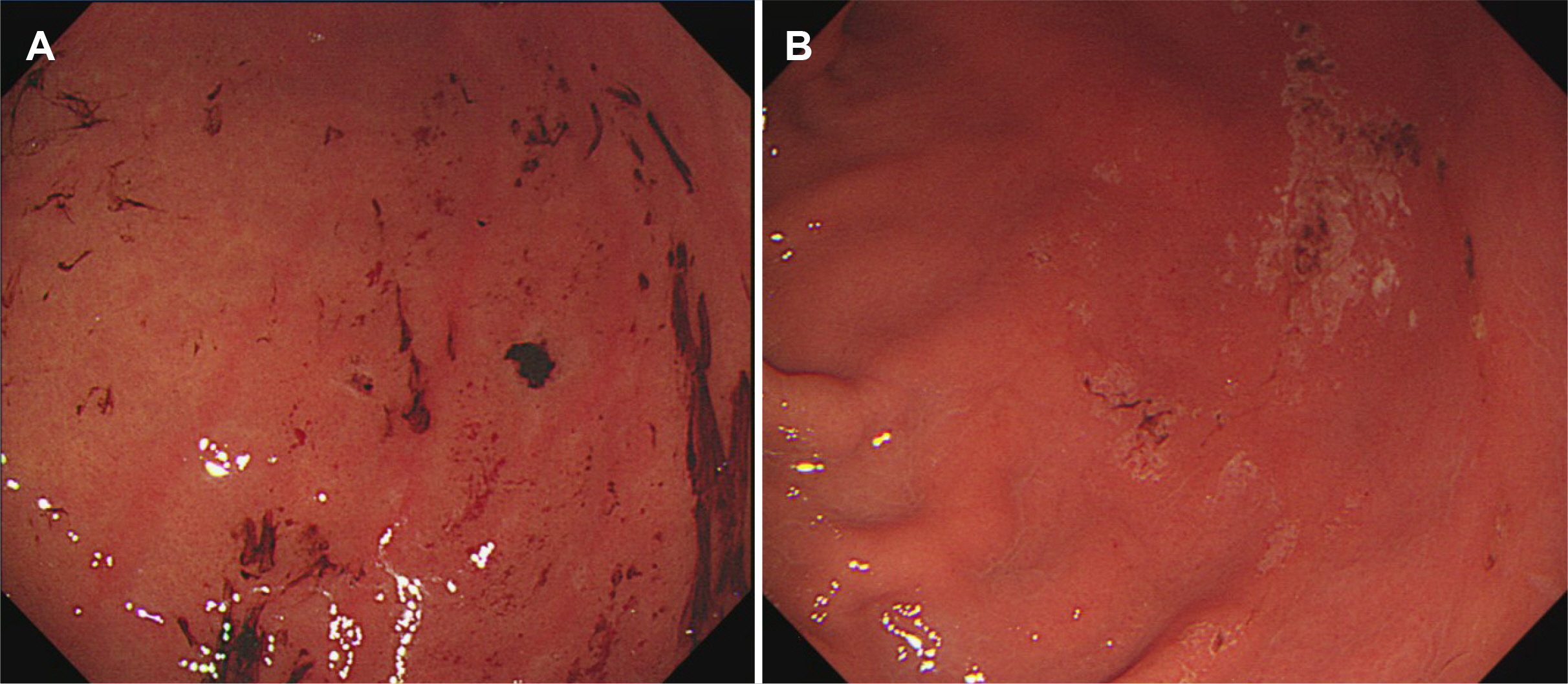
Comparison of the OST group (n=2,463) with the 1 L PEG/Asc group (n=2,060) revealed that the rates of successful cleansing and high-quality cleansing were similar between the two groups. Polyp and adenoma detection rates were significantly higher in the OST group than in the 1 L PEG/Asc group (p<0.001 and p=0.013), while the incidence of acute gastric mucosal lesion-like blood stain/clot, erosions at greater curvature side of antrum/body, multiple erosions, and overlying mucosal erythema or edema were all significantly higher in the OST group than in the 1 L PEG/Asc group (all p<0.001). Additionally, high and indeterminate probability scores of preparation agent-induced gastropathy (p=0.001) and mean Lanza scores were significantly higher in the OST group than in the 1 L PEG/Asc group (1.3 vs. 0.4, p<0.001).
Conclusions
Compared with 1 L PEG/Asc, OSTs were significantly associated with acute gastropathy during bowel preparation, thus requiring careful consideration from physicians for the simultaneous screening of EGD and colonoscopy.
Figure
Reference
-
1. Nishihara R, Wu K, Lochhead P, et al. 2013; Long-term colorectal-cancer incidence and mortality after lower endoscopy. N Engl J Med. 369:1095–1105. DOI: 10.1056/NEJMoa1301969. PMID: 24047059. PMCID: PMC3840160.2. Løberg M, Kalager M, Holme Ø, Hoff G, Adami HO, Bretthauer M. 2014; Long-term colorectal-cancer mortality after adenoma removal. N Engl J Med. 371:799–807. DOI: 10.1056/NEJMoa1315870. PMID: 25162886.3. Cohen LB, Sanyal SM, Von Althann C, et al. 2010; Clinical trial: 2-L polyethylene glycol-based lavage solutions for colonoscopy preparation- a randomized, single-blind study of two formulations. Aliment Pharmacol Ther. 32:637–644. DOI: 10.1111/j.1365-2036.2010.04390.x. PMID: 20626383.4. Hassan C, East J, Radaelli F, et al. 2019; Bowel preparation for colonoscopy: European Society of Gastrointestinal Endoscopy (ESGE) guideline - update 2019. Endoscopy. 51:775–794. DOI: 10.1055/a-0959-0505. PMID: 31295746.5. Jung Y, Kang SB, Yoon HJ, Cha JM. 2022; Improving the tolerability and safety of 1-L polyethylene glycol plus low-dose ascorbic acid for bowel preparation in a healthy population: a randomized multicenter clinical trial. Gastrointest Endosc. 96:341–350.e1. DOI: 10.1016/j.gie.2022.03.007. PMID: 35288148.6. Yoon JY, Kim HG, Cho YS, Kim HI, Cha JM. 2022; 1 L- versus 2 L-polyethylene glycol with ascorbic acid for bowel preparation in elderly patients: a randomized multicenter study. Surg Endosc. 36:5724–5733. DOI: 10.1007/s00464-021-08947-4. PMID: 35031868.7. Di Palma JA, Bhandari R, Cleveland MV, et al. 2021; A safety and efficacy comparison of a new sulfate-based tablet bowel preparation versus a PEG and ascorbate comparator in adult subjects undergoing colonoscopy. Am J Gastroenterol. 116:319–328. DOI: 10.14309/ajg.0000000000001020. PMID: 33165006. PMCID: PMC7864663.8. Kim JH, Park YE, Kim TO, et al. Comparison of the efficacy and safety between oral sulfate tablet and polyethylene glycol for bowel preparation before colonoscopy according to age. Medicine (Baltimore). 2022; 101:e29884. DOI: 10.1097/MD.0000000000029884. PMID: 35801801. PMCID: PMC9259131.9. Lee SE, Oh DJ, Nam JH, et al. 2023; Taking oral sulfate tablets with simethicone for bowel preparation leads to higher adenoma detection rate than polyethylene glycol: a propensity score analysis. Dig Dis Sci. 68:867–876. DOI: 10.1007/s10620-022-07611-8. PMID: 35781655.10. Villa E, Bansal M, Zakko W. 2021; Oral sulfate tablet bowel preparation is associated with erosive gastritis. Am J Gastroenterol. 116:S263. DOI: 10.14309/01.ajg.0000774788.09911.e3.11. Yang HJ, Park DI, Park SK, et al. 2020; Novel sulfate tablet PBK-1701TC versus oral sulfate solution for colon cleansing: a randomized phase 3 trial. J Gastroenterol Hepatol. 35:29–36. DOI: 10.1111/jgh.14826. PMID: 31396995.12. Lai EJ, Calderwood AH, Doros G, Fix OK, Jacobson BC. 2009; The Boston bowel preparation scale: a valid and reliable instrument for colonoscopy-oriented research. Gastrointest Endosc. 69(3 Pt 2):620–625. DOI: 10.1016/j.gie.2008.05.057. PMID: 19136102. PMCID: PMC2763922.13. Calderwood AH, Jacobson BC. 2010; Comprehensive validation of the Boston Bowel Preparation Scale. Gastrointest Endosc. 72:686–692. DOI: 10.1016/j.gie.2010.06.068. PMID: 20883845. PMCID: PMC2951305.14. Kwak MS, Cha JM, Yang HJ, et al. 2019; Safety and efficacy of low-volume preparation in the elderly: oral sulfate solution on the day before and split-dose regimens (SEE SAFE) study. Gut Liver. 13:176–182. DOI: 10.5009/gnl18214. PMID: 30400725. PMCID: PMC6430430.15. Hagège H, Laugier R, Nahon S, Coulom P, Isnard-Bagnis C, Albert-Marty A. 2015; Real-life conditions of use of sodium phosphate tablets for colon cleansing before colonoscopy. Endosc Int Open. 3:E346–E353. DOI: 10.1055/s-0034-1391847. PMID: 26357680. PMCID: PMC4554515.16. McGettigan MJ, Menias CO, Gao ZJ, Mellnick VM, Hara AK. 2016; Imaging of drug-induced complications in the gastrointestinal system. Radiographics. 36:71–87. DOI: 10.1148/rg.2016150132. PMID: 26761532.17. Lanza FL, Collaku A, Liu DJ. 2018; Endoscopic comparison of gastroduodenal injury with over-the-counter doses of new fast-dissolving ibuprofen and paracetamol formulations: a randomized, placebo-controlled, 4-way crossover clinical trial. Clin Exp Gastroenterol. 11:169–177. DOI: 10.2147/CEG.S153231. PMID: 29713191. PMCID: PMC5907787.18. Coron E, Dewitte M, Aubert P, Musquer N, Neunlist M, Bruley des Varannes S. 2015; Reversibility of gastric mucosal lesions induced by sodium phosphate tablets and characterized by probe-based confocal laser endomicroscopy. Endosc Int Open. 3:E69–E75. DOI: 10.1055/s-0034-1377934. PMID: 26134776. PMCID: PMC4423282.19. Park JH, Hong SW, Hwang SW, et al. 2023; Efficacy and safety of oral sodium sulfate tablet compared with 1-L polyethylene glycol plus ascorbate: a prospective, randomized, endoscopist-blinded trial. J Gastroenterol Hepatol. 38:2090–2096. DOI: 10.1111/jgh.16343. PMID: 37655723.20. Sacks NC, Sharma A, Cyr PL, Bertiger G, Dahdal DN, Brogadir SP. 2018; Real-world comparison of the effectiveness and safety of different bowel preparation agents. Clin Exp Gastroenterol. 11:289–299. DOI: 10.2147/CEG.S171861. PMID: 30555250. PMCID: PMC6280884.21. Pyenson B, Scammell C, Broulette J. 2014; Costs and repeat rates associated with colonoscopy observed in medical claims for commercial and medicare populations. BMC Health Serv Res. 14:92. DOI: 10.1186/1472-6963-14-92. PMID: 24572047. PMCID: PMC3974039.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Acute Gastropathy Associated with Bowel Preparation According to Age: Oral Sulfate Tablets versus 1-L Polyethylene Glycol with Ascorbic Acid
- Predictors of Inadequate Bowel Preparation and Salvage Options on Colonoscopy
- Optimal and Safe Bowel Preparation for Colonoscopy
- Safe and appropriate use of laxatives for colonoscopy
- Bowel Preparation for Surveillance Colonoscopy After Colorectal Resection: A New Perspective