J Pathol Transl Med.
2024 Nov;58(6):299-309. 10.4132/jptm.2024.07.25.
Diagnostic challenges in the assessment of thyroid neoplasms using nuclear features and vascular and capsular invasion: a multi-center interobserver agreement study
- Affiliations
-
- 1Department of Anatomical Pathology, Faculty of Medicine, Universitas Indonesia/Dr. Cipto Mangunkusumo Hospital, Jakarta, Indonesia
- 2Human Cancer Research Center-Indonesian Medical Education and Research Institute, Faculty of Medicine, Universitas Indonesia, Jakarta, Indonesia
- 3Faculty of Medicine, Universitas Muhammadiyah Palembang, Palembang, Indonesia
- 4Faculty of Medicine, Universitas Padjadjaran/Hasan Sadikin General Hospital, Bandung, Indonesia
- 5Department of Otorhinolaryngology, Head and Neck Surgery, Harapan Kita National Women and Children Health Center, Jakarta, Indonesia
- 6Department of Anatomical Pathology, Faculty of Medicine, Hasanuddin University, Makassar, Indonesia
- 7Department of Anatomical Pathology, Faculty of Medicine, Andalas University, Padang, Indonesia
- 8Department of Anatomical Pathology, Faculty of Medicine, Universitas Brawijaya/RSUD dr. Saiful Anwar, Malang, Indonesia
- 9Department of Anatomical Pathology, Faculty of Medicine and Health Science, Universitas Jambi, Jambi, Indonesia
- 10Department of Anatomical Pathology, Faculty of Medicine, Universitas Padjadjaran/Hasan Sadikin General Hospital, Bandung, Indonesia
- 11Department of Anatomical Pathology, Faculty of Medicine, Universitas Diponegoro, Semarang, Indonesia
- 12Department of Anatomical Pathology, Faculty of Medicine, University of Lampung, Lampung, Indonesia
- 13Department of Anatomical Pathology, Faculty of Medicine, University of Sriwijaya, Palembang, Indonesia
- 14Department of Anatomical Pathology, Labuha Hospital, South Halmahera, Indonesia
- 15Department of Anatomical Pathology, Faculty of Medicine, Universitas Udayana, Prof. Dr. I.G.N.G. Ngoerah Hospital, Denpasar, Indonesia
- 16Department of Anatomical Pathology, Faculty of Medicine, Universitas Airlangga/Dr Soetomo Academic Hospital, Surabaya, Indonesia
- 17Department of Anatomical Pathology, Faculty of Medicine, Public Health, and Nursing, Universitas Gadjah Mada/UGM Academic Hospital, Yogyakarta, Indonesia
- 18Department of Anatomical Pathology, Dr. Ben Mboi Hospital, Kupang, Indonesia
- 19Kanujoso Djatiwibowo Hospital, Balikpapan, Indonesia
- 20Department of Pathology and Thyroid Disease Center, Izumi City General Hospital, Izumi, Japan
- KMID: 2560671
- DOI: http://doi.org/10.4132/jptm.2024.07.25
Abstract
- Background
The diagnosis of thyroid neoplasms necessitates the identification of distinct histological features. Various education/hospital centers located in cities across Indonesia likely result in discordances among pathologists when diagnosing thyroid neoplasms.
Methods
This study examined the concordance among Indonesian pathologists in assessing nuclear features and capsular and vascular invasion of thyroid tumors. Fifteen pathologists from different centers independently assessed the same 14 digital slides of thyroid tumor specimens. All the specimens were thyroid neoplasms with known BRAFV600E and RAS mutational status, from a single center. We evaluated the pre- and post-training agreement using the Fleiss kappa. The significance of the training was evaluated using a paired T-test.
Results
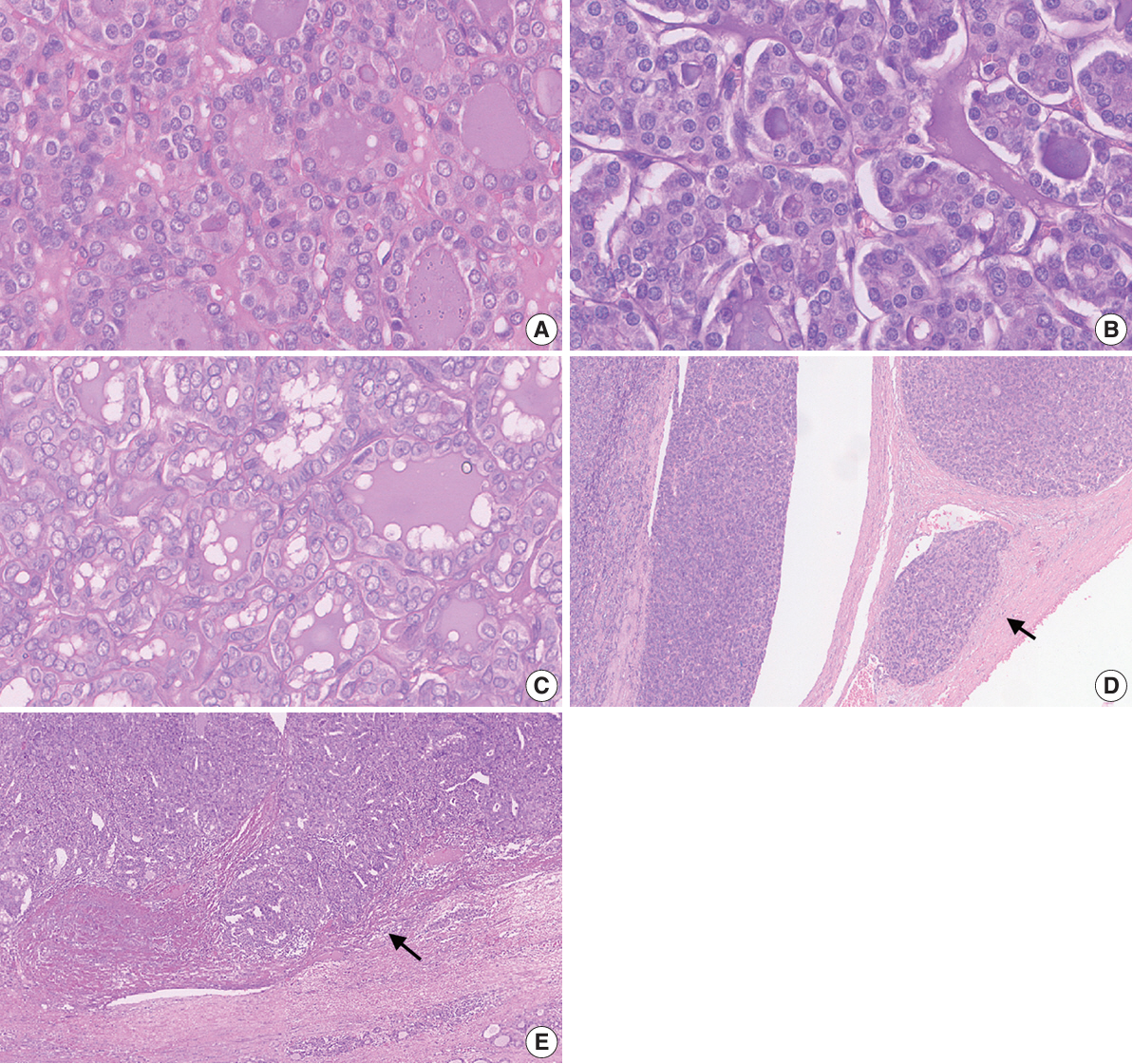
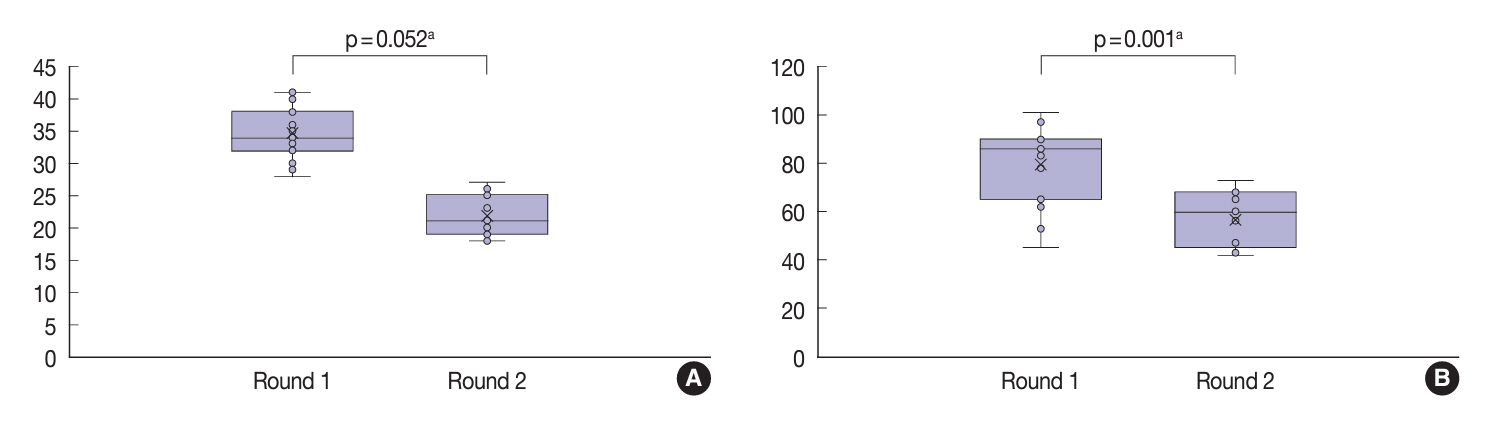
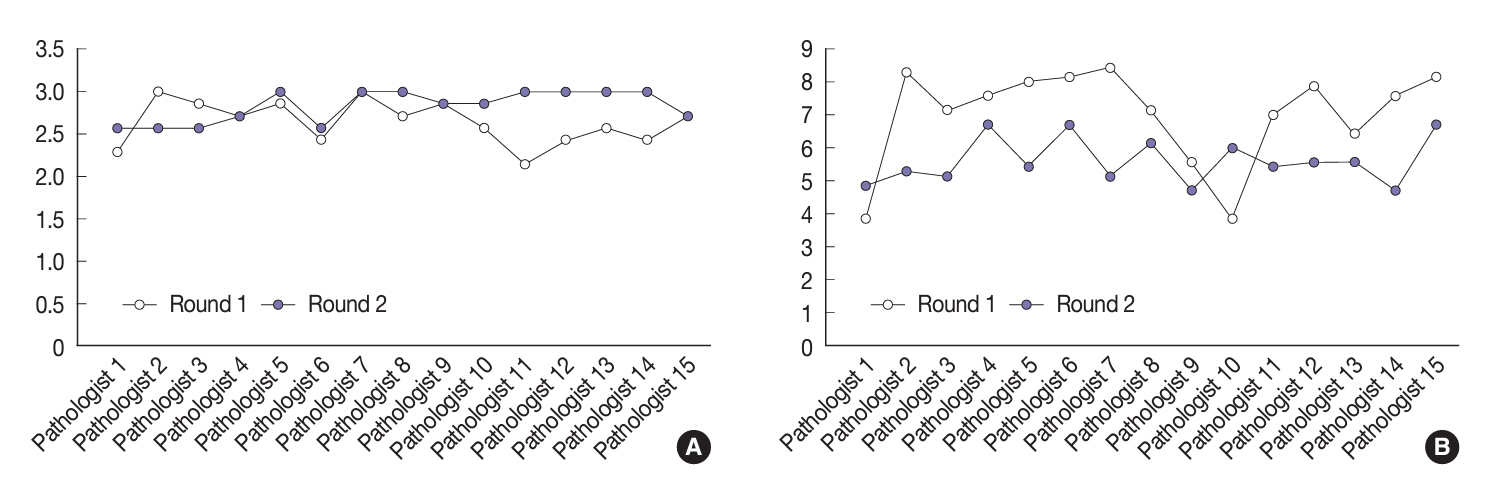
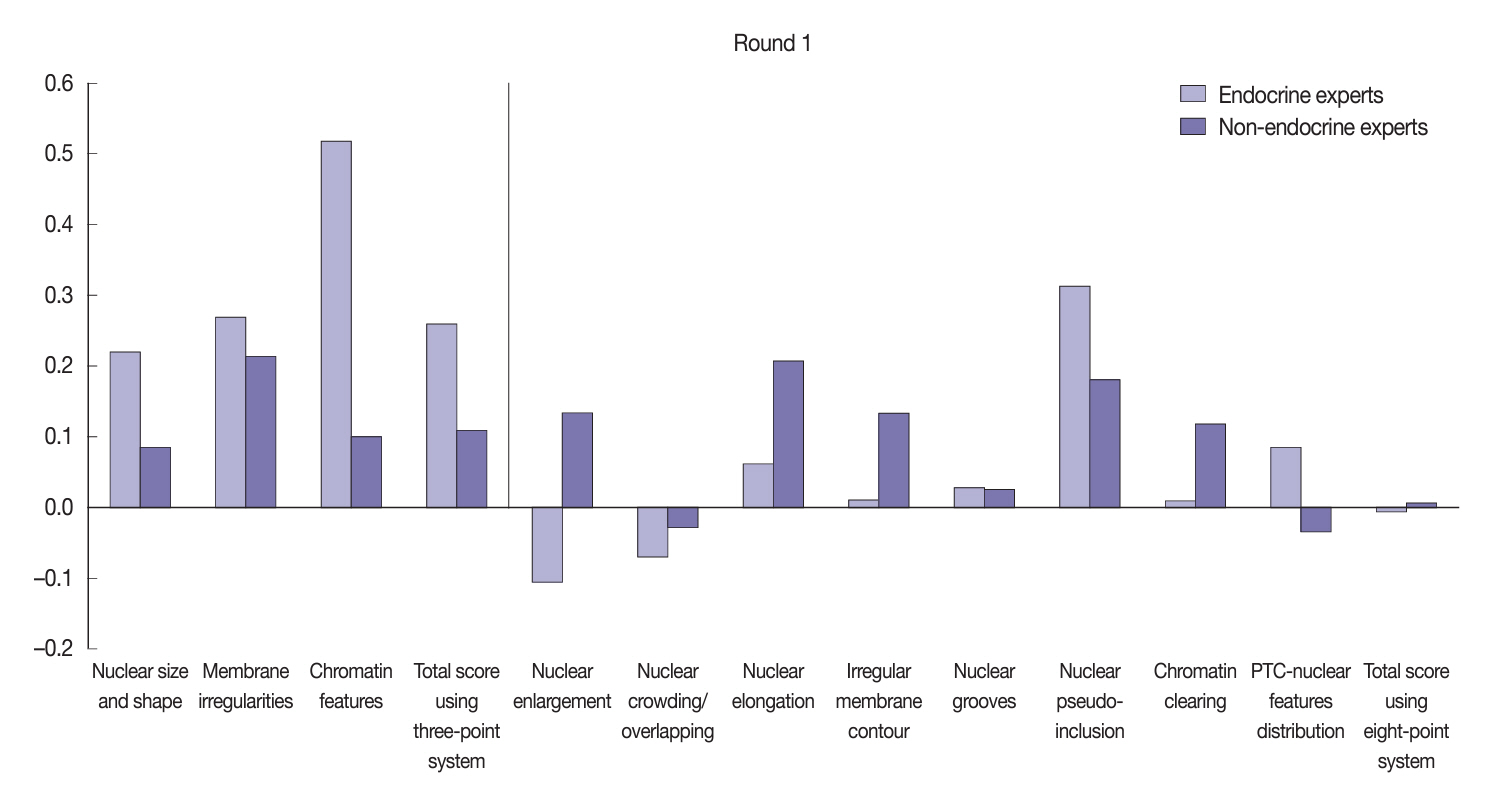
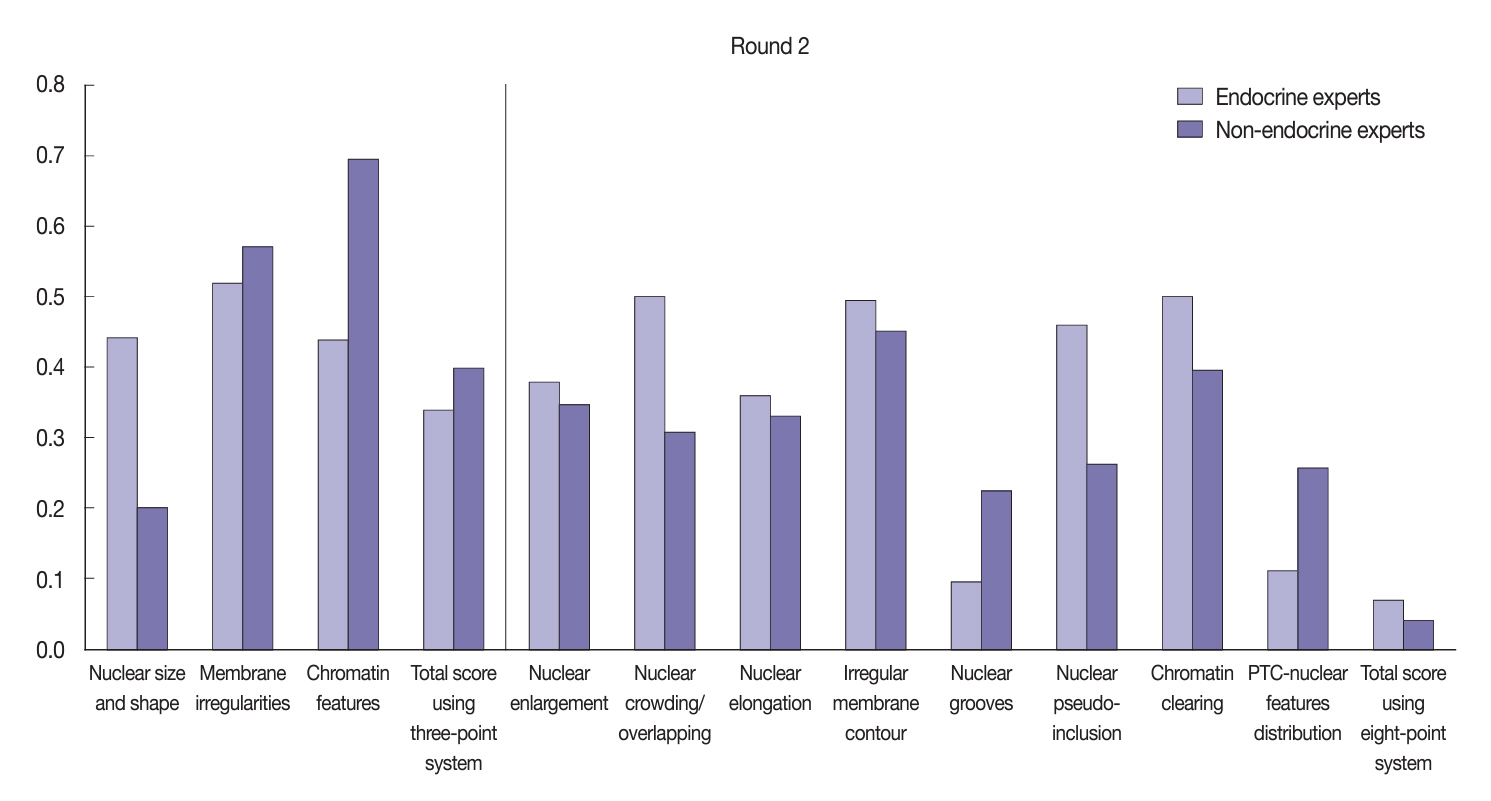
Baseline agreement on nuclear features was slight to fair based on a 3-point scoring system (k = 0.14 to 0.28) and poor to fair based on an eight-point system (k = –0.02 to 0.24). Agreements on vascular (κ = 0.35) and capsular invasion (κ = 0.27) were fair, whereas the estimated molecular type showed substantial agreement (κ = 0.74). Following the training, agreement using the eight-point system significantly improved (p = 0.001).
Conclusions
The level of concordance among Indonesian pathologists in diagnosing thyroid neoplasm was relatively poor. Consensus in pathology assessment requires ongoing collaboration and education to refine diagnostic criteria.
Figure
Reference
-
References
1. Bray F, Laversanne M, Sung H, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024; 74:229–63.
Article2. Baloch ZW, Mete O, Fadda G, et al. Papillary thyroid carcinoma. WHO classification of tumours series, 5th ed, Vol. 10. Endocrine and neuroendocrine tumours [Internet]. Lyon: International Agency for Research on Cancer;2022. [cited 2024 Jul 15]. Available from: https://tumourclassification.iarc.who.int/chapters/53.3. Lim H, Devesa SS, Sosa JA, Check D, Kitahara CM. Trends in thyroid cancer incidence and mortality in the United States, 1974-2013. JAMA. 2017; 317:1338–48.
Article4. Wiltshire JJ, Drake TM, Uttley L, Balasubramanian SP. Systematic review of trends in the incidence rates of thyroid cancer. Thyroid. 2016; 26:1541–52.
Article5. Donnelly D, Geoghegan R, O’Brien C, Philbin E, Wheeler TS. Synthesis of heterocyclic-substituted chromones and related compounds as potential anticancer agents. J Med Chem. 1965; 8:872–5.6. Liu Z, Bychkov A, Jung CK, et al. Interobserver and intraobserver variation in the morphological evaluation of noninvasive follicular thyroid neoplasm with papillary-like nuclear features in Asian practice. Pathol Int. 2019; 69:202–10.
Article7. Thompson LD, Poller DN, Kakudo K, Burchette R, Nikiforov YE, Seethala RR. An international interobserver variability reporting of the nuclear scoring criteria to diagnose noninvasive follicular thyroid neoplasm with papillary-like nuclear features: a validation study. Endocr Pathol. 2018; 29:242–9.
Article8. Hirokawa M, Carney JA, Goellner JR, et al. Observer variation of encapsulated follicular lesions of the thyroid gland. Am J Surg Pathol. 2002; 26:1508–14.
Article9. Elsheikh TM, Asa SL, Chan JK, et al. Interobserver and intraobserver variation among experts in the diagnosis of thyroid follicular lesions with borderline nuclear features of papillary carcinoma. Am J Clin Pathol. 2008; 130:736–44.
Article10. Lloyd RV, Erickson LA, Casey MB, et al. Observer variation in the diagnosis of follicular variant of papillary thyroid carcinoma. Am J Surg Pathol. 2004; 28:1336–40.
Article11. Zhu Y, Li Y, Jung CK, et al. Histopathologic assessment of capsular invasion in follicular thyroid neoplasms: an observer variation study. Endocr Pathol. 2020; 31:132–40.
Article12. Su HK, Wenig BM, Haser GC, et al. Inter-observer variation in the pathologic identification of minimal extrathyroidal extension in papillary thyroid carcinoma. Thyroid. 2016; 26:512–7.
Article13. Nikiforov YE, Seethala RR, Tallini G, et al. Nomenclature revision for encapsulated follicular variant of papillary thyroid carcinoma: a paradigm shift to reduce overtreatment of indolent tumors. JAMA Oncol. 2016; 2:1023–9.
Article14. Jung CK, Bychkov A, Song DE, et al. Molecular correlates and nuclear features of encapsulated follicular-patterned thyroid neoplasms. Endocrinol Metab (Seoul). 2021; 36:123–33.
Article15. Harahap AS, Subekti I, Panigoro SS, et al. Profile of BRAFV600E, BRAFK601E, NRAS, HRAS, and KRAS mutational status, and clinicopathological characteristics of papillary thyroid carcinoma in Indonesian National Referral Hospital. Appl Clin Genet. 2023; 16:99–110.
Article16. Xin Y, Guan D, Meng K, Lv Z, Chen B. Diagnostic accuracy of CK-19, Galectin-3 and HBME-1 on papillary thyroid carcinoma: a meta-analysis. Int J Clin Exp Pathol. 2017; 10:8130–40.17. Morariu EM, McCoy KL, Chiosea SI, et al. Clinicopathologic characteristics of thyroid nodules positive for the THADA-IGF2BP3 fusion on preoperative molecular analysis. Thyroid. 2021; 31:1212–8.
Article18. Chernock RD, Rivera B, Borrelli N, et al. Poorly differentiated thyroid carcinoma of childhood and adolescence: a distinct entity characterized by DICER1 mutations. Mod Pathol. 2020; 33:1264–74.
Article19. Seethala RR, Baloch ZW, Barletta JA, et al. Noninvasive follicular thyroid neoplasm with papillary-like nuclear features: a review for pathologists. Mod Pathol. 2018; 31:39–55.
Article20. Farmer M, Petras RE, Hunt LE, Janosky JE, Galandiuk S. The importance of diagnostic accuracy in colonic inflammatory bowel disease. Am J Gastroenterol. 2000; 95:3184–8.
Article21. Kerkhof M, van Dekken H, Steyerberg EW, et al. Grading of dysplasia in Barrett’s oesophagus: substantial interobserver variation between general and gastrointestinal pathologists. Histopathology. 2007; 50:920–7.
Article22. Xu B, Wang L, Tuttle RM, Ganly I, Ghossein R. Prognostic impact of extent of vascular invasion in low-grade encapsulated follicular cell-derived thyroid carcinomas: a clinicopathologic study of 276 cases. Hum Pathol. 2015; 46:1789–98.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Erratum: Diagnostic challenges in the assessment of thyroid neoplasms using nuclear features and vascular and capsular invasion: a multi-center interobserver agreement study
- Interobserver Agreement among Experts in Determining the Depth of Invasion of Early Colorectal Carcinoma
- Histologic Degree of Invasion and Prognosis in Follicular Thyroid Carcinoma
- Molecular Correlates and Nuclear Features of Encapsulated Follicular-Patterned Thyroid Neoplasms
- Preoperative Diagnosis of Extraglandular Invasion of Thyroid Papillary Carcinoma: High Resolution Sonography versus Multidetector Computed Tomography