Intest Res.
2024 Oct;22(4):439-452. 10.5217/ir.2023.00175.
Defining management strategies for acute severe ulcerative colitis using predictive models: a simulation-modeling study
- Affiliations
-
- 1Department of Gastroenterology, Austin Health, Heidelberg, Australia
- 2Department of Medicine, Faculty of Medicine, Dentistry & Health Sciences, The University of Melbourne, Parkville, Australia
- KMID: 2560294
- DOI: http://doi.org/10.5217/ir.2023.00175
Abstract
- Background/Aims
Robust management algorithms are required to reduce the residual risk of colectomy in acute severe ulcerative colitis (ASUC) refractory to standard infliximab salvage therapy. The aim of this study was to evaluate the performance and benefits of alternative ASUC management strategies using simulated prediction models of varying accuracy.
Methods
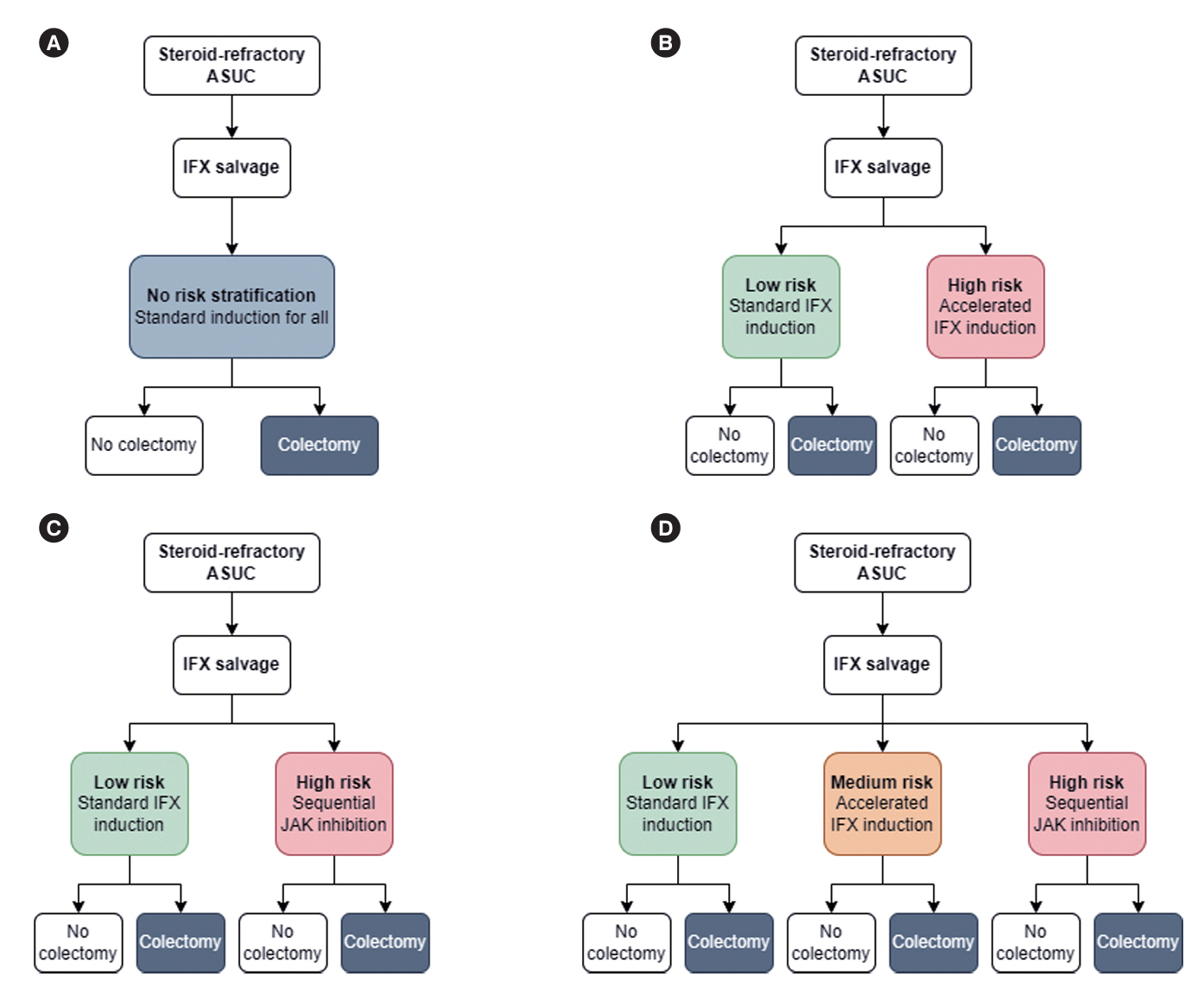
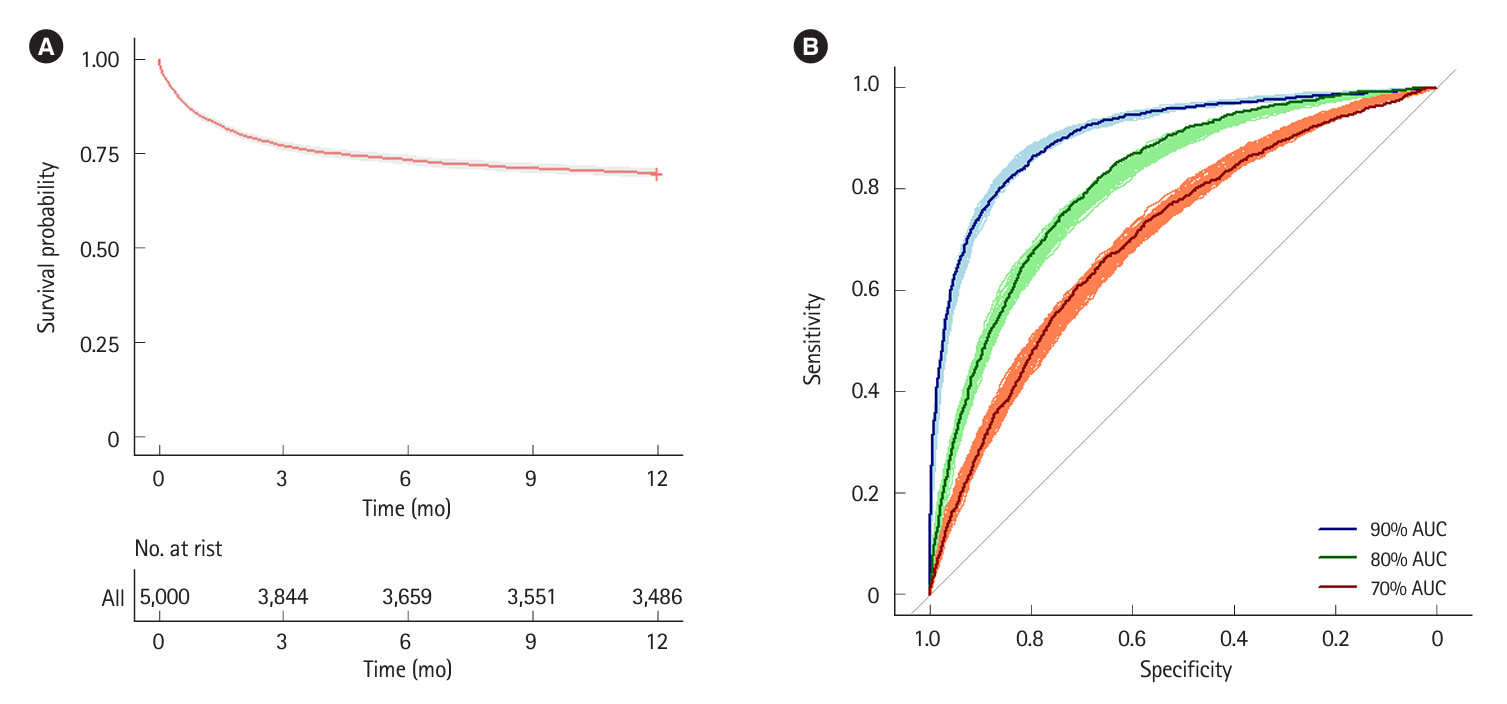
This was a simulation-based modeling study using a hypothetical cohort of 5,000 steroid-refractory ASUC patients receiving standard infliximab induction. Simulated predictive models were used to risk-stratify patients and escalate treatment in patients at high risk of failing standard infliximab induction. The main outcome of interest was colectomy by 3 months.
Results
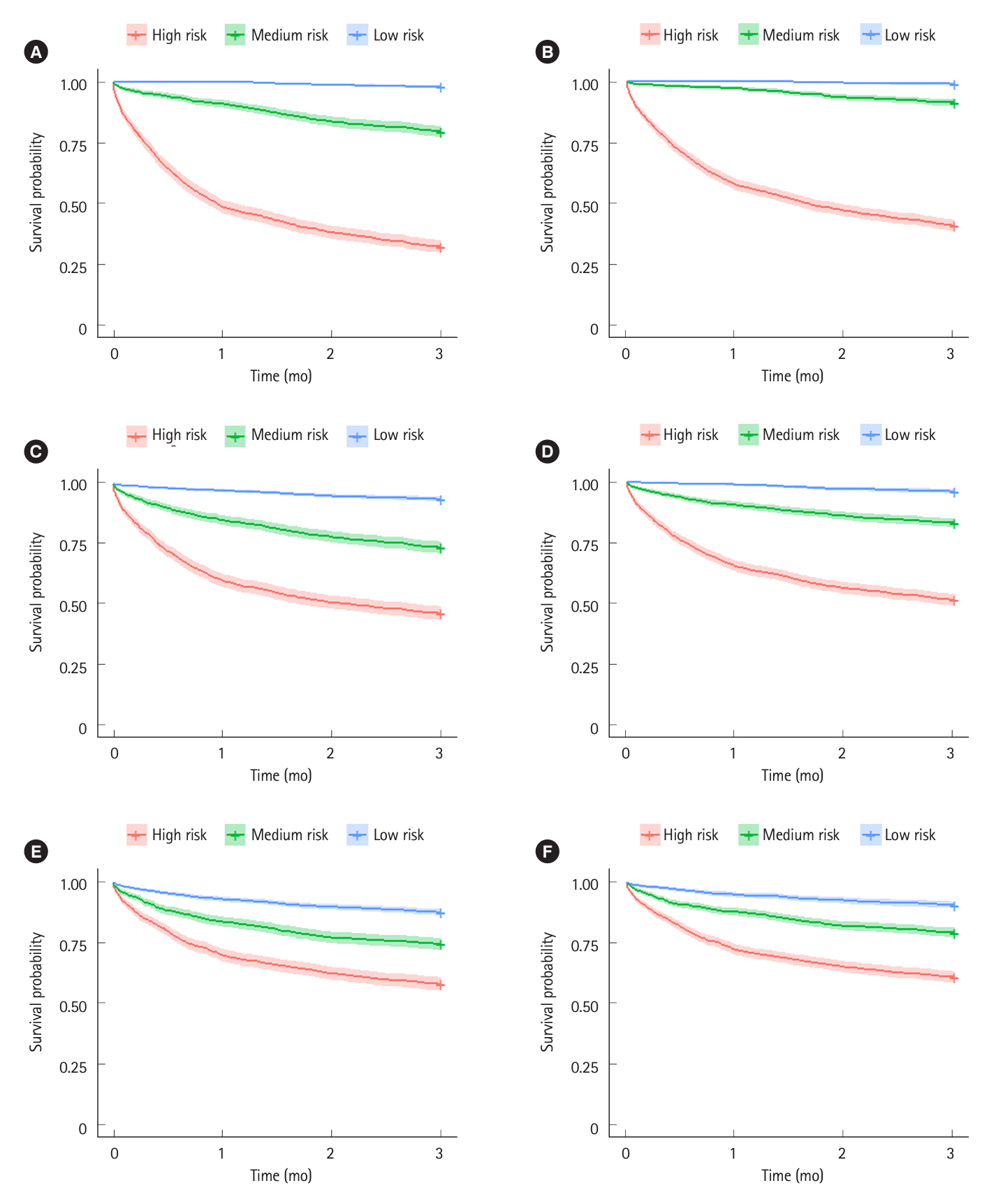
The 3-month colectomy rate in the base scenario where all 5,000 patients received standard infliximab induction was 23%. The best-performing management strategy assigned high-risk patients to sequential Janus kinase inhibitor inhibition and mediumrisk patients to accelerated infliximab induction. Using a 90% area under the curve (AUC) prediction model and optimistic treatment efficacy assumptions, this strategy reduced the 3-month colectomy rate to 8% (65% residual risk reduction). Using an 80% AUC prediction model with only modest treatment efficacy assumptions, the 3-month colectomy rate was reduced to 15% (35% residual risk reduction). Overall management strategy efficacy was highly dependent on predictive model accuracy and underlying treatment efficacy assumptions.
Conclusions
This is the first study to simulate predictive model-based management strategies in steroid-refractory ASUC and evaluate their effect on short-term colectomy rates. Future studies on predictive model development should incorporate simulation studies to better understand their expected benefit.
Figure
Reference
-
1. Wang R, Li Z, Liu S, Zhang D. Global, regional and national burden of inflammatory bowel disease in 204 countries and territories from 1990 to 2019: a systematic analysis based on the Global Burden of Disease Study 2019. BMJ Open. 2023; 13:e065186.
Article2. Seah D, De Cruz P. Review article: the practical management of acute severe ulcerative colitis. Aliment Pharmacol Ther. 2016; 43:482–513.
Article3. Choy MC, Seah D, Faleck DM, et al. Systematic review and meta-analysis: optimal salvage therapy in acute severe ulcerative colitis. Inflamm Bowel Dis. 2019; 25:1169–1186.
Article4. Sebastian S, Myers S, Argyriou K, et al. Infliximab induction regimens in steroid-refractory acute severe colitis: a multicentre retrospective cohort study with propensity score analysis. Aliment Pharmacol Ther. 2019; 50:675–683.
Article5. Hindryckx P, Novak G, Vande Casteele N, et al. Review article: dose optimisation of infliximab for acute severe ulcerative colitis. Aliment Pharmacol Ther. 2017; 45:617–630.6. Gilmore R, Tan WL, Fernandes R, An YK, Begun J. Upadacitinib salvage therapy for infliximab-experienced patients with acute severe ulcerative colitis. J Crohns Colitis. 2023; 17:2033–2036.
Article7. Steenholdt C, Dige Ovesen P, Brynskov J, Benedict Seidelin J. Tofacitinib for acute severe ulcerative colitis: a systematic review. J Crohns Colitis. 2023; 17:1354–1363.
Article8. Zinger CH, Ringel Y, Eitan M, et al. Upadacitinib for acute severe ulcerative colitis. Inflamm Bowel Dis. 2023; 29:1667–1669.
Article9. Vieujean S, Louis E. Precision medicine and drug optimization in adult inflammatory bowel disease patients. Therap Adv Gastroenterol. 2023; 16:17562848231173331.
Article10. Cheah E, Huang JG. Precision medicine in inflammatory bowel disease: individualizing the use of biologics and small molecule therapies. World J Gastroenterol. 2023; 29:1539–1550.11. Con D, Parthasarathy N, Bishara M, et al. Development of a simple, serum biomarker-based model predictive of the need for early biologic therapy in Crohn’s disease. J Crohns Colitis. 2021; 15:583–593.
Article12. Con D, van Langenberg DR, Vasudevan A. Deep learning vs conventional learning algorithms for clinical prediction in Crohn’s disease: a proof-of-concept study. World J Gastroenterol. 2021; 27:6476–6488.
Article13. Stafford IS, Gosink MM, Mossotto E, Ennis S, Hauben M. A systematic review of artificial intelligence and machine learning applications to inflammatory bowel disease, with practical guidelines for interpretation. Inflamm Bowel Dis. 2022; 28:1573–1583.
Article14. Syal G, Robbins L, Kashani A, et al. Hypoalbuminemia and bandemia predict failure of infliximab rescue therapy in acute severe ulcerative colitis. Dig Dis Sci. 2021; 66:199–205.
Article15. Gibson DJ, Doherty J, McNally M, et al. Comparison of medium to long-term outcomes of acute severe ulcerative colitis patients receiving accelerated and standard infliximab induction. Frontline Gastroenterol. 2019; 11:441–447.16. Chao CY, Al Khoury A, Aruljothy A, et al. High-dose infliximab rescue therapy for hospitalized acute severe ulcerative colitis does not improve colectomy-free survival. Dig Dis Sci. 2019; 64:518–523.
Article17. Nalagatla N, Falloon K, Tran G, et al. Effect of accelerated infliximab induction on short- and long-term outcomes of acute severe ulcerative colitis: a retrospective multicenter study and meta-analysis. Clin Gastroenterol Hepatol. 2019; 17:502–509.
Article18. Shah SC, Naymagon S, Panchal HJ, Sands BE, Cohen BL, Dubinsky MC. Accelerated infliximab dosing increases 30-day colectomy in hospitalized ulcerative colitis patients: a propensity score analysis. Inflamm Bowel Dis. 2018; 24:651–659.
Article19. Gibson DJ, Heetun ZS, Redmond CE, et al. An accelerated infliximab induction regimen reduces the need for early colectomy in patients with acute severe ulcerative colitis. Clin Gastroenterol Hepatol. 2015; 13:330–335.
Article20. Uzzan M, Bresteau C, Laharie D, et al. Tofacitinib as salvage therapy for 55 patients hospitalised with refractory severe ulcerative colitis: a GETAID cohort. Aliment Pharmacol Ther. 2021; 54:312–319.21. Berinstein JA, Sheehan JL, Dias M, et al. Tofacitinib for biologic-experienced hospitalized patients with acute severe ulcerative colitis: a retrospective case-control study. Clin Gastroenterol Hepatol. 2021; 19:2112–2120.
Article22. Eqbal A, Hilley P, Choy M, Srinivasan A, de Cruz P. Outcomes out to 12 months after sequential use of high-dose tofacitinib following infliximab in acute severe ulcerative colitis. Intern Med J. 2023; 53:1497–1500.
Article23. Constant BD, Baldassano R, Kirsch J, Mitchel EB, Stein R, Albenberg L. Tofacitinib salvage therapy for children hospitalized for corticosteroid- and biologic-refractory ulcerative colitis. J Pediatr Gastroenterol Nutr. 2022; 75:724–730.
Article24. Xiao Y, Benoit N, Sedano R, et al. Effectiveness of tofacitinib for hospitalized patients with acute severe ulcerative colitis: case series. Dig Dis Sci. 2022; 67:5213–5219.
Article25. Komeda Y, Kono M, Kashida H, et al. Successful initial tofacitinib treatment for acute severe ulcerative colitis with steroid resistance: a case series. Ann Gastroenterol. 2023; 36:97–102.26. Malakar S, Kothalkar S, Shamsul Hoda U, Ghoshal UC. Tofacitinib in steroid-refractory acute severe ulcerative colitis: a retrospective analysis. Cureus. 2023; 15:e45416.
Article27. Honap S, Pavlidis P, Ray S, et al. Tofacitinib in acute severe ulcerative colitis: a real-world tertiary center experience. Inflamm Bowel Dis. 2020; 26:e147–e149.28. Gilmore R, Hilley P, Srinivasan A, Choy M, De Cruz P. Sequential use of high-dose tofacitinib after infliximab salvage therapy in acute severe ulcerative colitis. J Crohns Colitis. 2022; 16:166–168.
Article29. Kotwani P, Terdiman J, Lewin S. Tofacitinib for rescue therapy in acute severe ulcerative colitis: a real-world experience. J Crohns Colitis. 2020; 14:1026–1028.
Article30. Ali NM, Shehab MA. Upadacitinib as a rescue therapy in acute severe ulcerative colitis: a case report and review of the literature. Am J Case Rep. 2023; 24:e940966.31. Zhang Z. Parametric regression model for survival data: Weibull regression model as an example. Ann Transl Med. 2016; 4:484.
Article32. Choy MC, Seah D, Gorelik A, et al. Predicting response after infliximab salvage in acute severe ulcerative colitis. J Gastroenterol Hepatol. 2018; 33:1347–1352.
Article33. Con D, Andrew B, Nicolaides S, van Langenberg DR, Vasudevan A. Biomarker dynamics during infliximab salvage for acute severe ulcerative colitis: C-reactive protein (CRP)-lymphocyte ratio and CRP-albumin ratio are useful in predicting colectomy. Intest Res. 2022; 20:101–113.
Article34. Grant RK, Jones GR, Plevris N, et al. The ACE (albumin, CRP and endoscopy) index in acute colitis: a simple clinical index on admission that predicts outcome in patients with acute ulcerative colitis. Inflamm Bowel Dis. 2021; 27:451–457.
Article35. Rosen MJ, Minar P, Vinks AA. Review article: applying pharmacokinetics to optimise dosing of anti-TNF biologics in acute severe ulcerative colitis. Aliment Pharmacol Ther. 2015; 41:1094–1103.36. Sandborn WJ, Su C, Sands BE, et al. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2017; 376:1723–1736.
Article37. Battat R, Hemperly A, Truong S, et al. Baseline clearance of infliximab is associated with requirement for colectomy in patients with acute severe ulcerative colitis. Clin Gastroenterol Hepatol. 2021; 19:511–518.
Article38. Con D, Vasudevan A, van Langenberg DR. Predictive scores in acute severe ulcerative colitis: which, what, and when are the decision points we should target? Clin Gastroenterol Hepatol. 2022; 20:e344–e345.
Article39. Weisshof R, Ollech JE, El Jurdi K, et al. Ciclosporin therapy after infliximab failure in hospitalized patients with acute severe colitis is effective and safe. J Crohns Colitis. 2019; 13:1105–1110.
Article40. Con D, Vasudevan A. Real-world guidance from artificial intelligence? Predicting outcomes of inflammatory bowel disease using machine learning. Dig Dis Sci. 2022; 67:4604–4605.41. Choy MC, Visvanathan K, De Cruz P. An overview of the innate and adaptive immune system in inflammatory bowel disease. Inflamm Bowel Dis. 2017; 23:2–13.42. Kantasiripitak W, Wicha SG, Thomas D, et al. A model-based tool for guiding infliximab induction dosing to maximize longterm deep remission in children with inflammatory bowel diseases. J Crohns Colitis. 2023; 17:896–908.
Article43. Liu XY, Tang H, Zhou QY, et al. Advancing the precision management of inflammatory bowel disease in the era of omics approaches and new technology. World J Gastroenterol. 2023; 29:272–285.
Article44. Stidham RW, Takenaka K. Artificial intelligence for disease assessment in inflammatory bowel disease: how will it change our practice? Gastroenterology. 2022; 162:1493–1506.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- What to do when traditional rescue therapies fail in acute severe ulcerative colitis
- A Case of Acute Pancreatitis associated with Ulcerative Colitis
- Acute Severe Ulcerative Colitis: Optimal Strategies for Drug Therapy
- Comparison of Cell Proliferation between Chronic Ulcerative Colitisand Acute Self-limited Colitis
- Trends in the incidence of ulcerative colitis in Korea