Intest Res.
2024 Oct;22(4):428-438. 10.5217/ir.2024.00006.
Ulcerative colitis-associated neoplasms often harbor poor prognostic histologic components with low detection by biopsy
- Affiliations
-
- 1Division of Gastroenterology and Hepatology, Department of Internal Medicine, Keio University School of Medicine, Tokyo, Japan
- 2Center for Diagnostic and Therapeutic Endoscopy, Keio University School of Medicine, Tokyo, Japan
- 3Department of Pathology, Keio University School of Medicine, Tokyo, Japan
- 4Center for Preventive Medicine, Keio University School of Medicine, Tokyo, Japan
- KMID: 2560293
- DOI: http://doi.org/10.5217/ir.2024.00006
Abstract
- Background/Aims
Poorly differentiated adenocarcinoma, signet-ring cell carcinoma, and mucinous adenocarcinoma (por/sig/muc), which are considered to be histologic subtypes with a poor prognosis, occur more frequently with colitis-associated cancer than with sporadic tumors. However, their invasiveness and manifestations are unclear. This study aimed to determine the prevalence of the por/sig/muc component in ulcerative colitis-associated neoplasms (UCANs) and its association with invasiveness and to clarify its clinicohistologic and endoscopic features.
Methods
This retrospective observational study included patients diagnosed with ulcerative colitis-associated high-grade dysplasia or adenocarcinoma from 1997 to 2022 who were divided according to the presence or absence of a por/sig/muc component.
Results
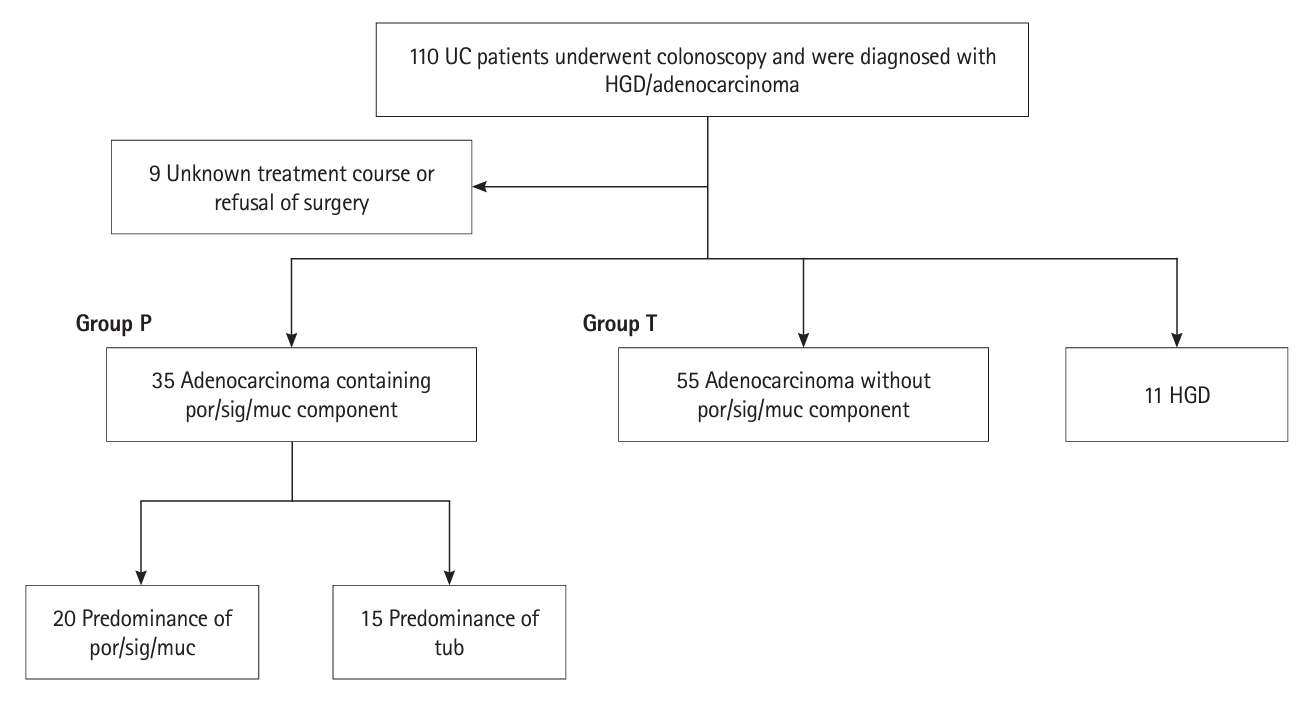
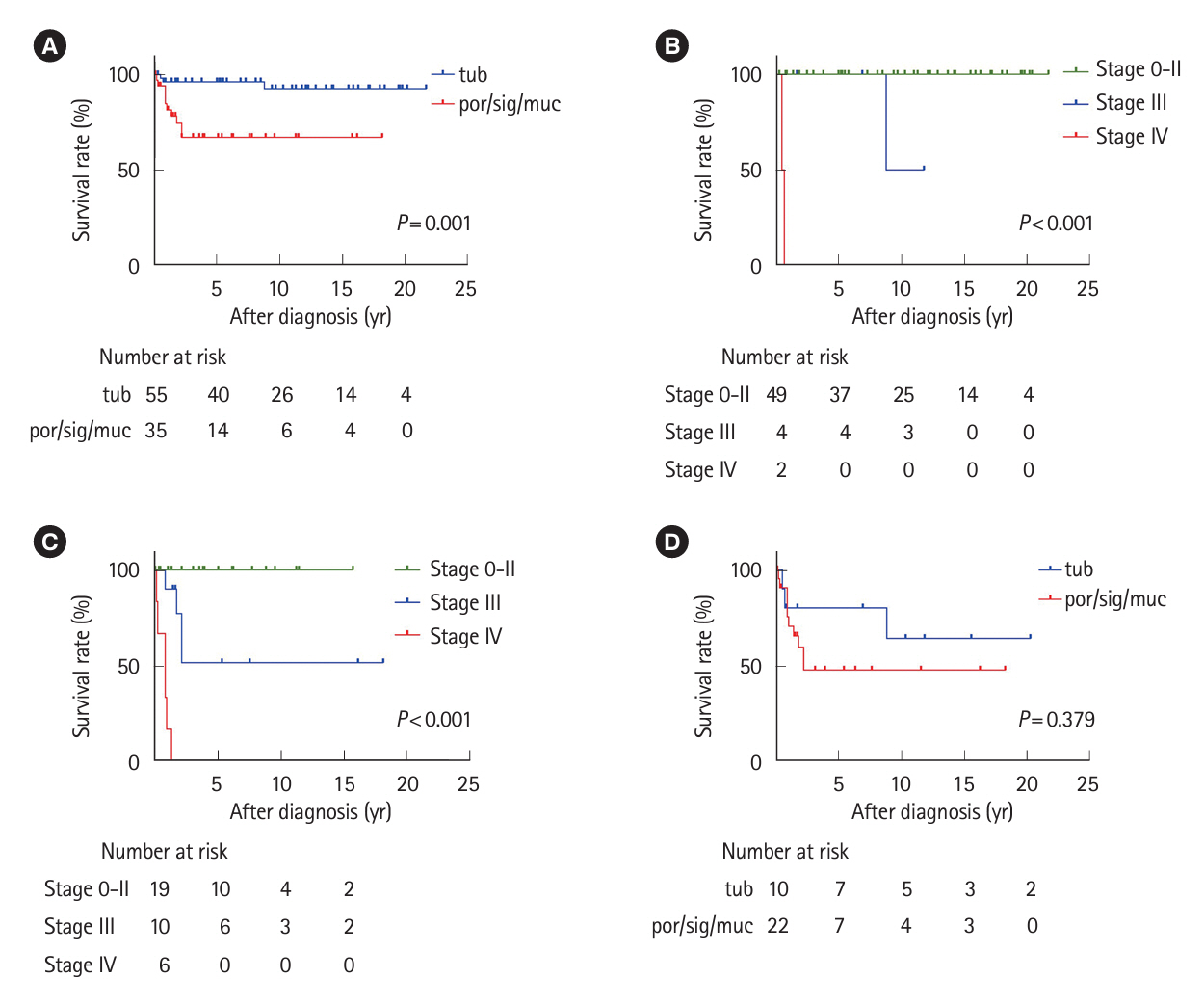
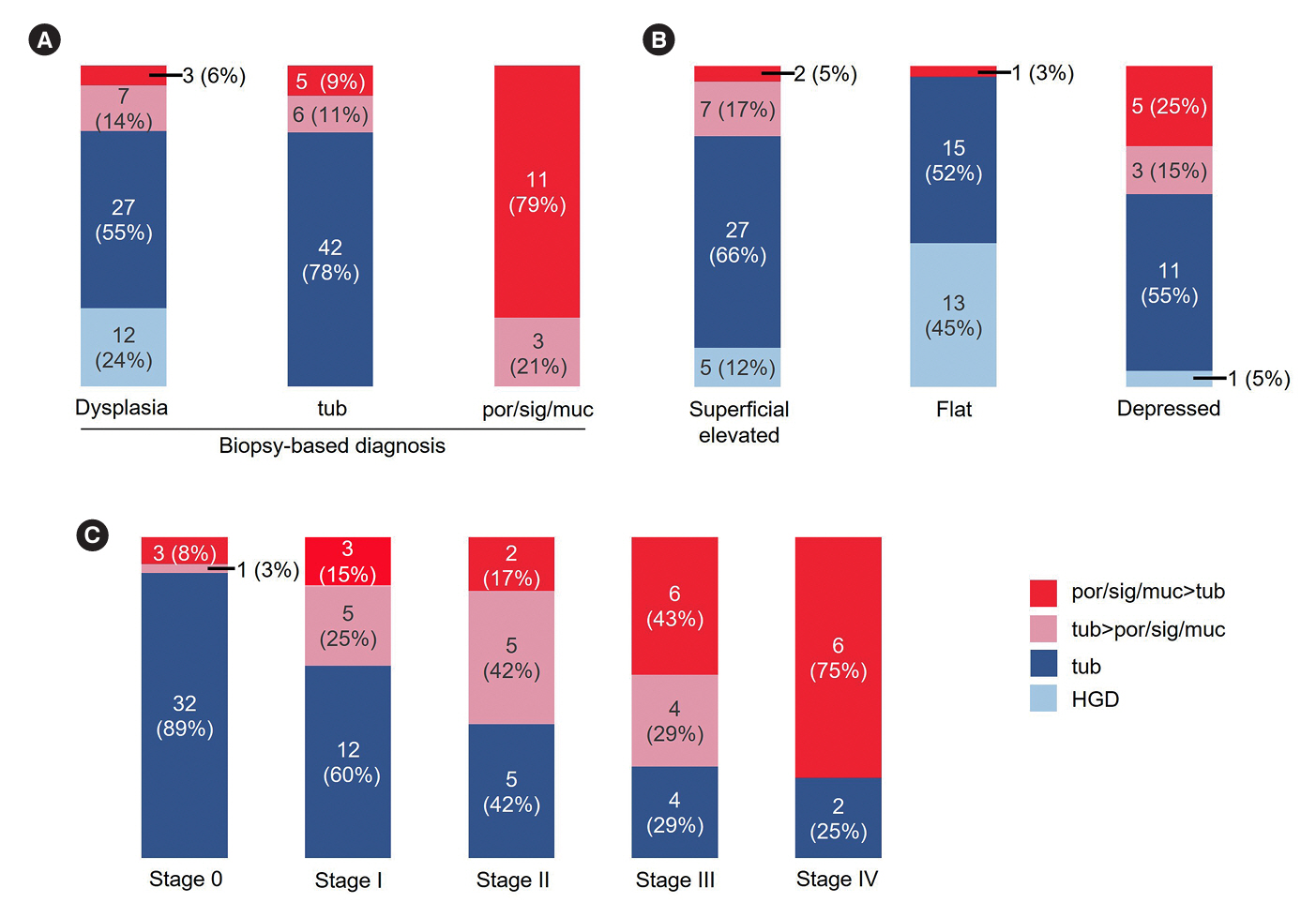
Thirty-five patients had UCAN with a por/sig/muc component and 66 had UCAN without this component. The 5-year survival rate was significantly lower in the por/sig/muc group than in the tub group (67% vs. 96%, P= 0.001), which was attributed to disease above stage III and depth to below the subserosa. Biopsy-based diagnosis before resection detected a por/sig/muc component in only 40% of lesions (14/35). Lesions with a por/sig/muc component were prevalent even in the early stages: stage 0 (4/36, 11%), I (8/20, 40%), II (7/12, 58%), III (10/14, 71%), and IV (6/8, 75%).
Conclusions
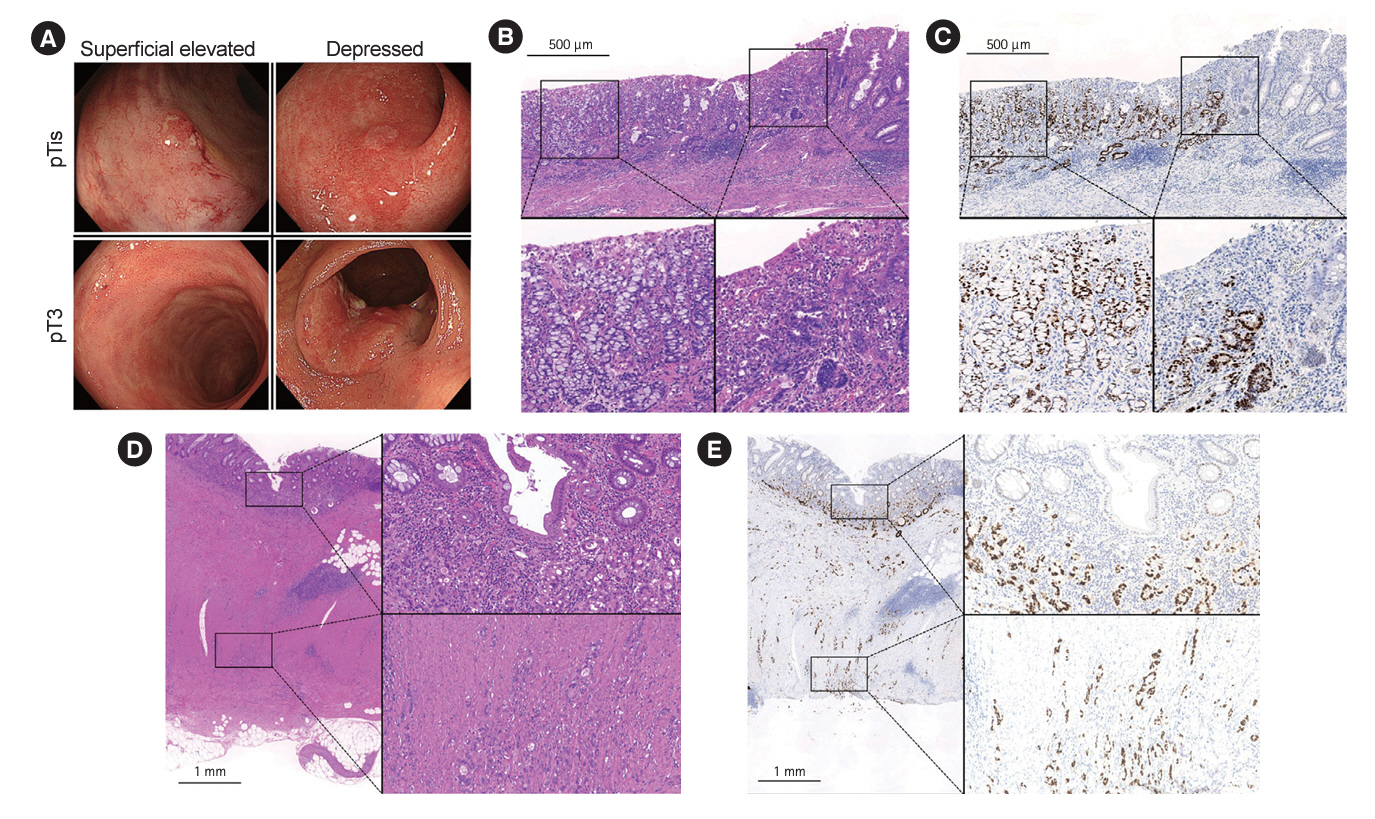
This is the first investigation that shows UCANs with a por/sig/muc component tended to be deeply invasive and were often not recognized preoperatively. Endoscopists should be aware that UCAN often has a por/sig/muc component that is not always recognized on biopsy, and the optimal treatment strategy needs to be carefully considered.
Figure
Reference
-
1. Turner D, Ricciuto A, Lewis A, et al. STRIDE-II: an update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): determining therapeutic goals for treat-to-target strategies in IBD. Gastroenterology. 2021; 160:1570–1583.
Article2. Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001; 48:526–535.3. Olén O, Erichsen R, Sachs MC, et al. Colorectal cancer in ulcerative colitis: a Scandinavian population-based cohort study. Lancet. 2020; 395:123–131.
Article4. Tsai L, Ma C, Dulai PS, et al. Contemporary risk of surgery in patients with ulcerative colitis and Crohn’s disease: a meta-analysis of population-based cohorts. Clin Gastroenterol Hepatol. 2021; 19:2031–2045.
Article5. Shah SC, Itzkowitz SH. Colorectal cancer in inflammatory bowel disease: mechanisms and management. Gastroenterology. 2022; 162:715–730.
Article6. Mir-Madjlessi SH, Farmer RG, Easley KA, Beck GJ. Colorectal and extracolonic malignancy in ulcerative colitis. Cancer. 1986; 58:1569–1574.
Article7. Lu C, Schardey J, Zhang T, et al. Survival outcomes and clinicopathological features in inflammatory bowel disease-associated colorectal cancer: a systematic review and meta-analysis. Ann Surg. 2022; 276:e319–e330.8. Sugimoto S, Shimoda M, Iwao Y, et al. Intramucosal poorly differentiated and signet-ring cell components in patients with ulcerative colitis-associated high-grade dysplasia. Dig Endosc. 2019; 31:706–711.
Article9. Watanabe T, Konishi T, Kishimoto J, et al. Ulcerative colitis-associated colorectal cancer shows a poorer survival than sporadic colorectal cancer: a nationwide Japanese study. Inflamm Bowel Dis. 2011; 17:802–808.
Article10. Laine L, Kaltenbach T, Barkun A, et al. SCENIC international consensus statement on surveillance and management of dysplasia in inflammatory bowel disease. Gastroenterology. 2015; 148:639–651.
Article11. Murthy SK, Feuerstein JD, Nguyen GC, Velayos FS. AGA clinical practice update on endoscopic surveillance and management of colorectal dysplasia in inflammatory bowel diseases: expert review. Gastroenterology. 2021; 161:1043–1051.
Article12. Gordon H, Biancone L, Fiorino G, et al. ECCO guidelines on inflammatory bowel disease and malignancies. J Crohns Colitis. 2023; 17:827–854.13. Ueno H, Mochizuki H, Hashiguchi Y, et al. Risk factors for an adverse outcome in early invasive colorectal carcinoma. Gastroenterology. 2004; 127:385–394.
Article14. Wang AY, Hwang JH, Bhatt A, Draganov PV. AGA clinical practice update on surveillance after pathologically curative endoscopic submucosal dissection of early gastrointestinal neoplasia in the United States: commentary. Gastroenterology. 2021; 161:2030–2040.
Article15. Sugimoto S, Naganuma M, Iwao Y, et al. Endoscopic morphologic features of ulcerative colitis-associated dysplasia classified according to the SCENIC consensus statement. Gastrointest Endosc. 2017; 85:639–646.16. Mutaguchi M, Naganuma M, Sugimoto S, et al. Difference in the clinical characteristic and prognosis of colitis-associated cancer and sporadic neoplasia in ulcerative colitis patients. Dig Liver Dis. 2019; 51:1257–1264.
Article17. Sugimoto S, Iwao Y, Shimoda M, et al. Epithelium replacement contributes to field expansion of squamous epithelium and ulcerative colitis-associated neoplasia. Gastroenterology. 2022; 162:334–337.18. Takabayashi K, Sugimoto S, Nanki K, et al. Characteristics of flat-type ulcerative colitis-associated neoplasia on chromoendoscopic imaging with indigo carmine dye spraying. Dig Endosc. 2024; 36:446–454.
Article19. Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis: a randomized study. N Engl J Med. 1987; 317:1625–1629.
Article20. Kobayashi S, Fujimori T, Mitomi H, et al. Immunohistochemical assessment of a unique basal pattern of p53 expression in ulcerative-colitis-associated neoplasia using computer-assisted cytometry. Diagn Pathol. 2014; 9:99.
Article21. Matkowskyj KA, Chen ZE, Rao MS, Yang GY. Dysplastic lesions in inflammatory bowel disease: molecular pathogenesis to morphology. Arch Pathol Lab Med. 2013; 137:338–350.
Article22. Japanese Society for Cancer of the Colon and Rectum. Japanese classification of colorectal, appendiceal, and anal carcinoma: the 3rd English edition. Tokyo: Kanehara & Co., Ltd.;2019.23. Kang H, O’Connell JB, Maggard MA, Sack J, Ko CY. A 10-year outcomes evaluation of mucinous and signet-ring cell carcinoma of the colon and rectum. Dis Colon Rectum. 2005; 48:1161–1168.24. Choi CR, Al Bakir I, Ding NJ, et al. Cumulative burden of inflammation predicts colorectal neoplasia risk in ulcerative colitis: a large single-centre study. Gut. 2019; 68:414–422.
Article25. Choi CH, Ignjatovic-Wilson A, Askari A, et al. Low-grade dysplasia in ulcerative colitis: risk factors for developing high-grade dysplasia or colorectal cancer. Am J Gastroenterol. 2015; 110:1461–1471.
Article26. Bak MTJ, Albéniz E, East JE, et al. Endoscopic management of patients with high-risk colorectal colitis-associated neoplasia: a Delphi study. Gastrointest Endosc. 2023; 97:767–779.
Article27. Mikami T, Yoshida T, Numata Y, et al. Invasive behavior of ulcerative colitis-associated carcinoma is related to reduced expression of CD44 extracellular domain: comparison with sporadic colon carcinoma. Diagn Pathol. 2011; 6:30.
Article28. Kaltenbach T, Holmes I, Nguyen-Vu T, et al. Longitudinal outcomes of the endoscopic resection of nonpolypoid dysplastic lesions in patients with inflammatory bowel disease. Gastrointest Endosc. 2023; 97:934–940.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Malignant Lymphoma in Patient with Ulcerative Colitis
- Can histologic remission be a better prognostic factor and therapeutic target beyond endoscopic mucosal healing in patients with ulcerative colitis?
- Recent Advances in Understanding Colorectal Cancer and Dysplasia Related to Ulcerative Colitis
- Histopathological Features of Endoscopic Biopsies in Ischemic Colitis
- Trends in the incidence of ulcerative colitis in Korea