Endocrinol Metab.
2024 Oct;39(5):793-802. 10.3803/EnM.2024.1999.
Subunit-Specific Developmental Roles of PI3K in SF1-Expressing Cells
- Affiliations
-
- 1Division of Physiology, Department of Oral Biology, Yonsei University College of Dentistry, Seoul, Korea
- 2Department of Global Medical Science, Yonsei University Wonju College of Medicine, Wonju, Korea
- 3Department of Applied Life Science, BK21 FOUR, Yonsei University College of Dentistry, Seoul, Korea
- KMID: 2560287
- DOI: http://doi.org/10.3803/EnM.2024.1999
Abstract
- Background
Phosphatidylinositol 3-kinase (PI3K) regulates cellular development and energy homeostasis. However, the roles of its subunits in organ development remain largely unknown.
Methods
We explored the roles of PI3K catalytic subunits in steroidogenic factor 1 (SF1)-expressing cells through knockout (KO) of the p110α and p110β subunits.
Results
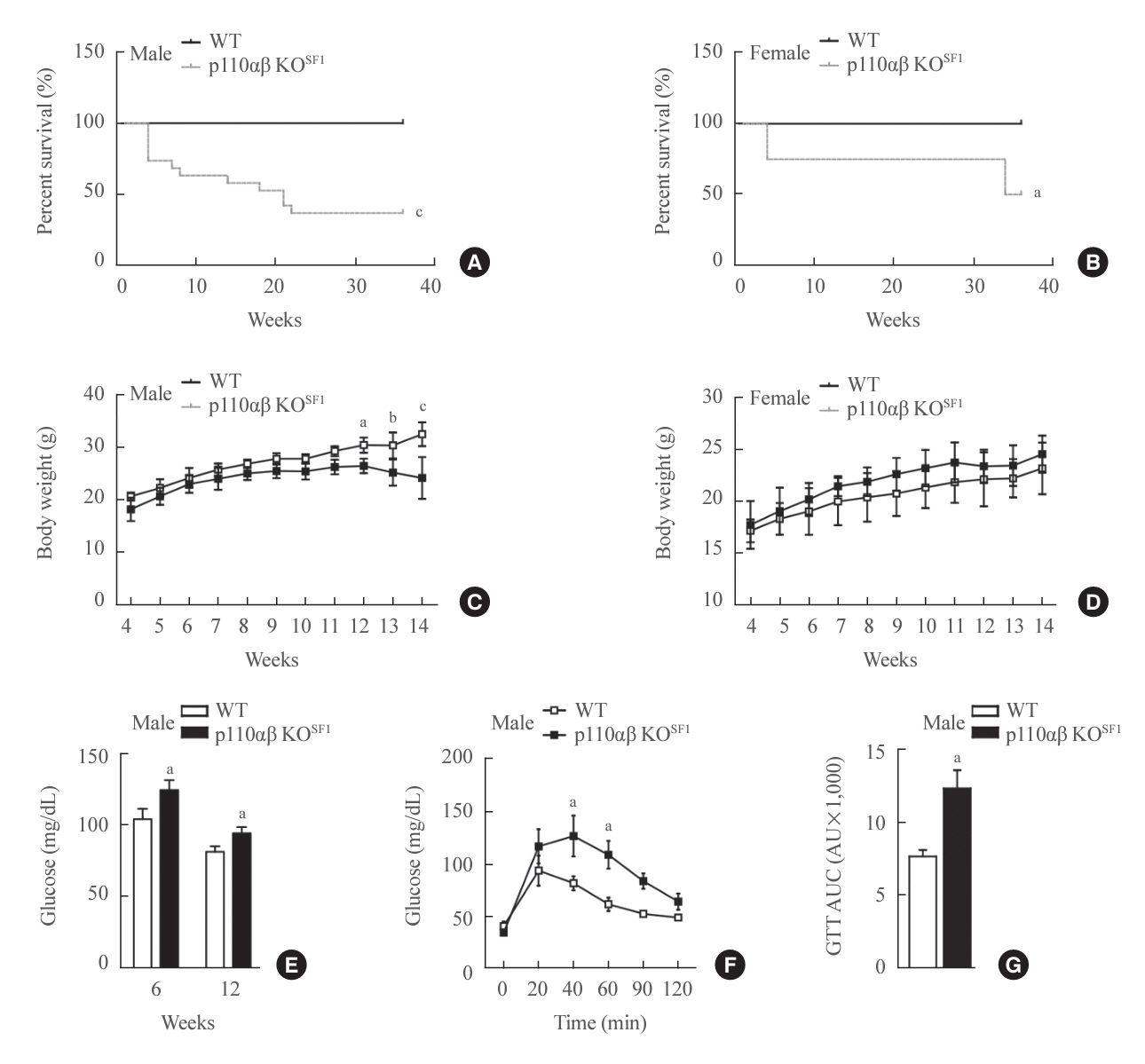
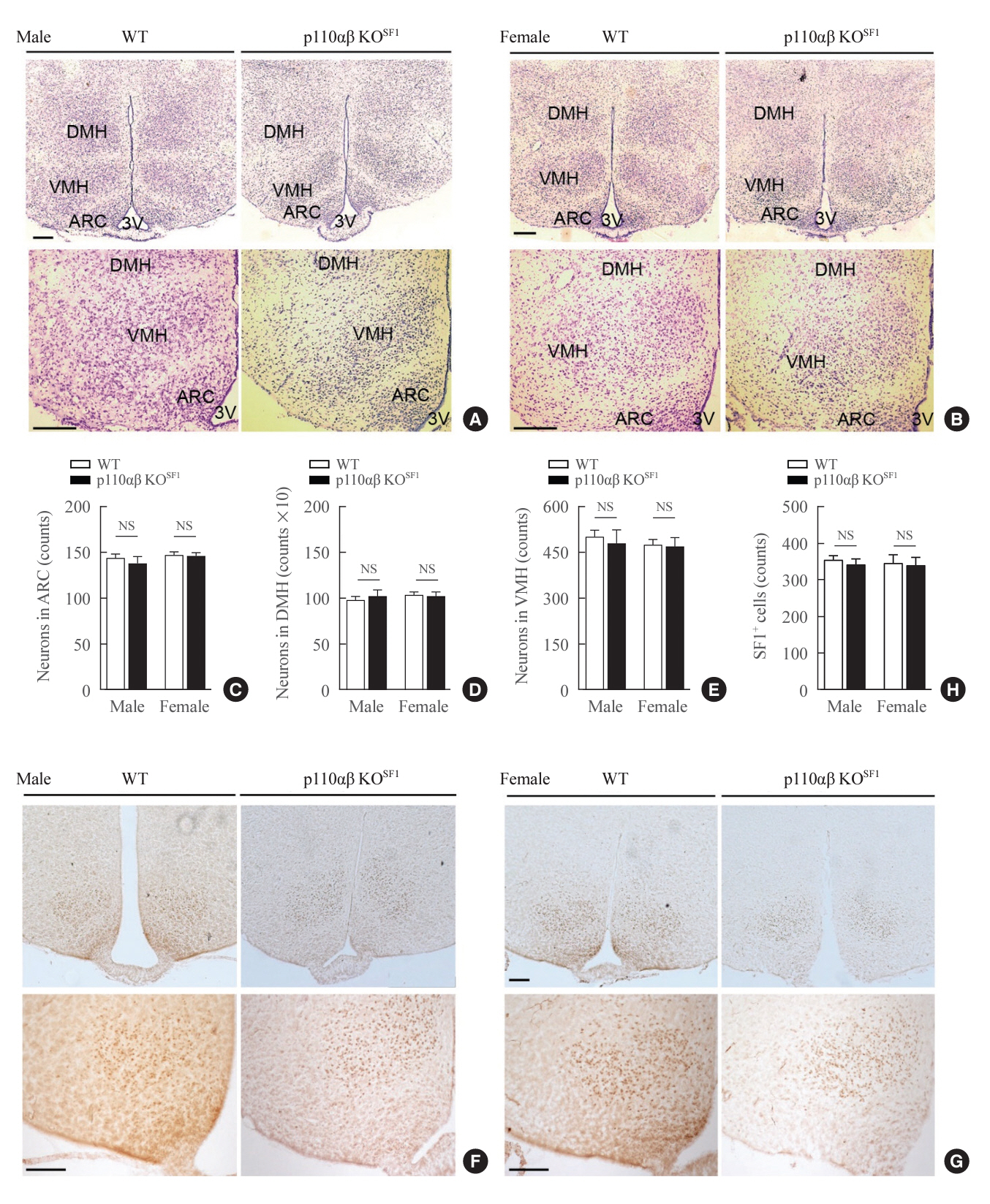
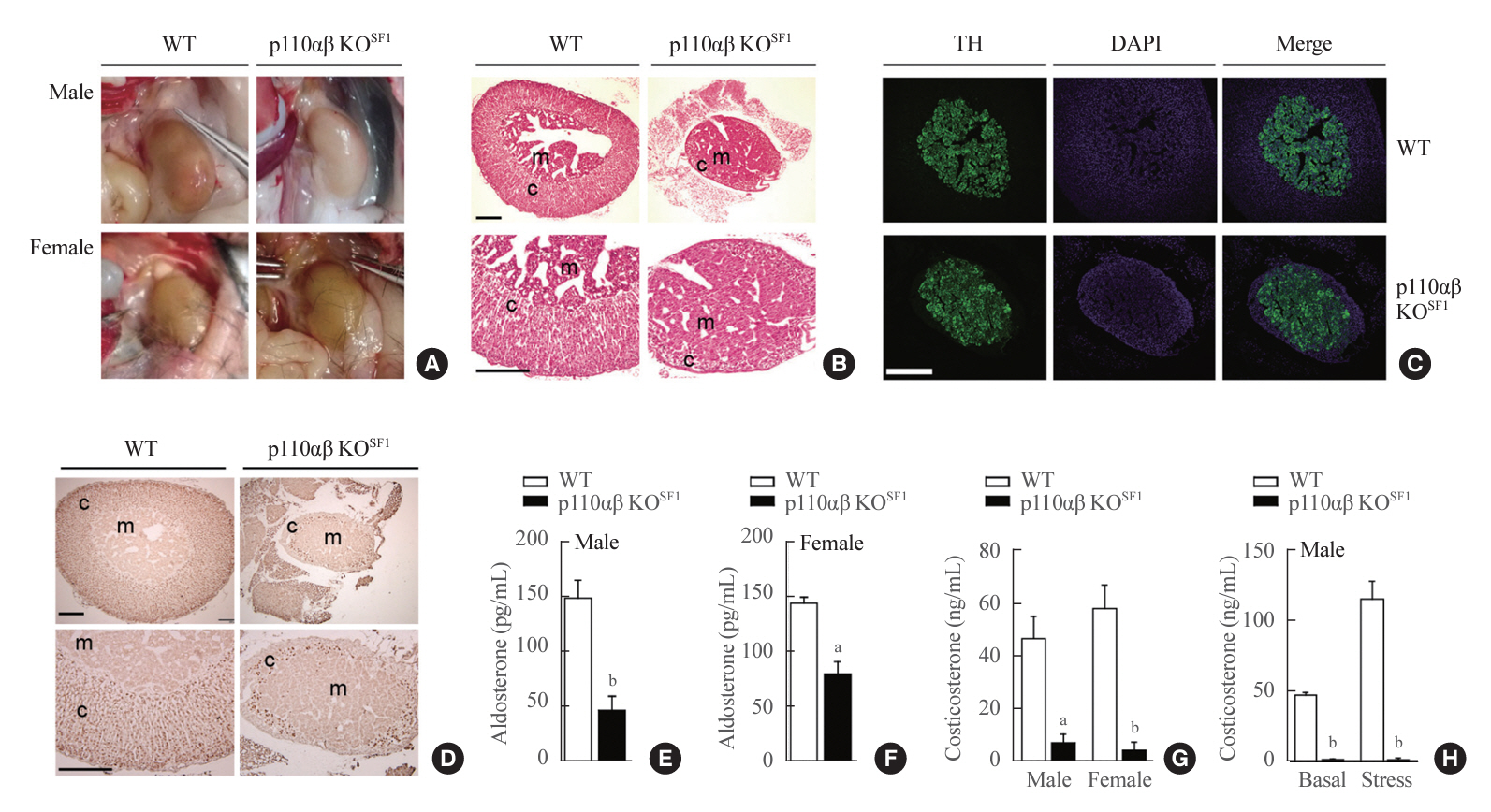
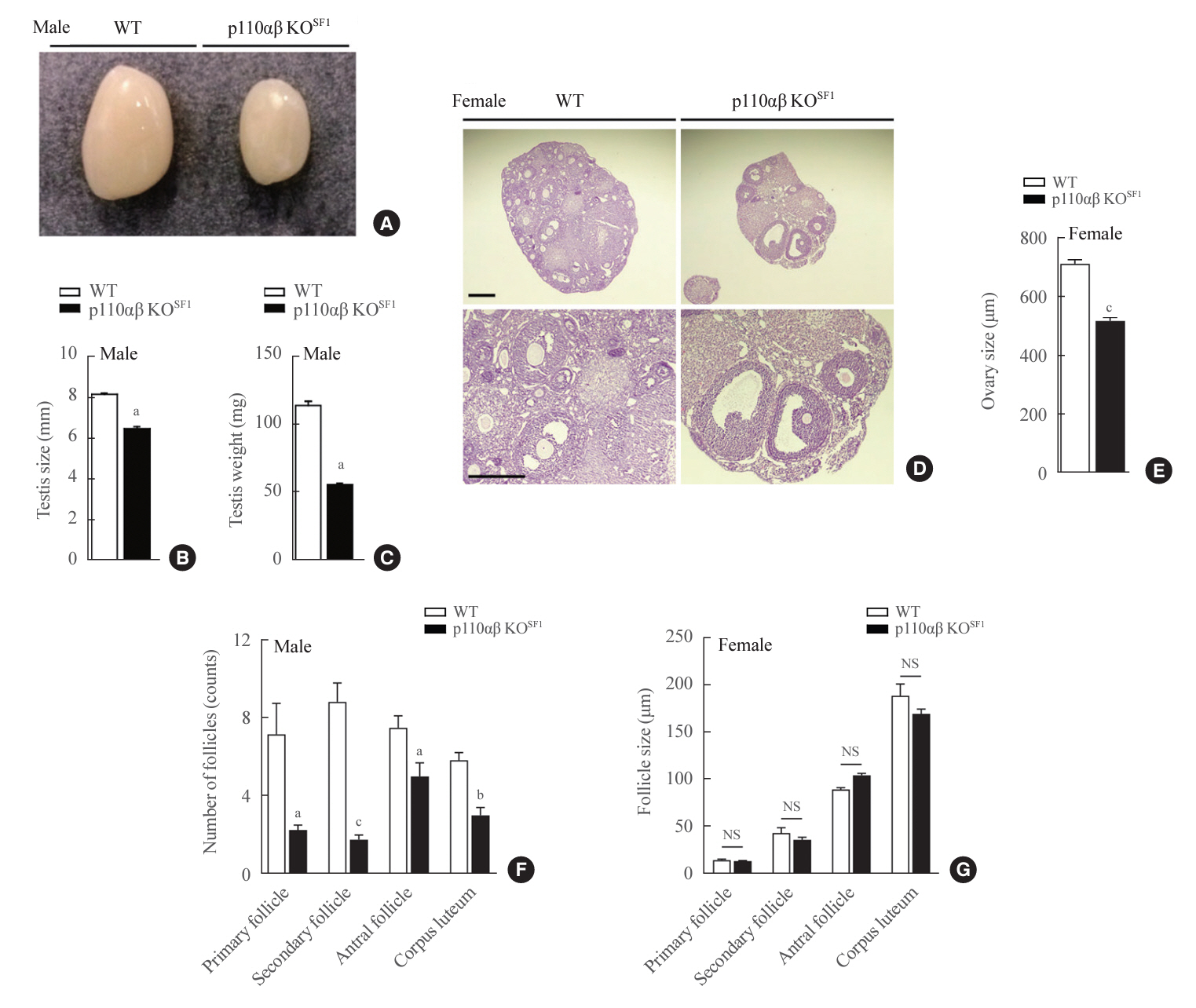
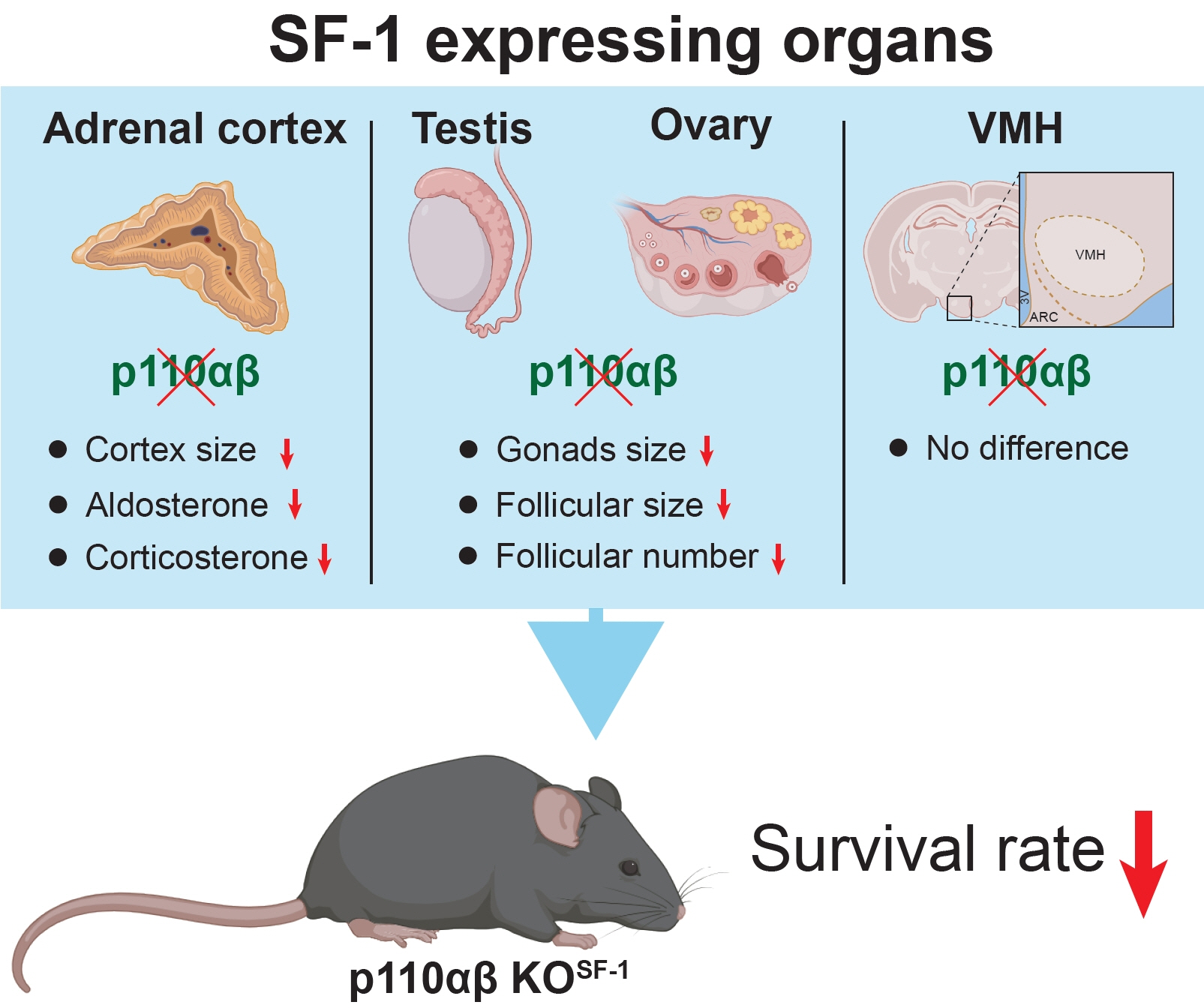
We examined mice with a double KO of p110α and p110β in SF1-expressing cells (p110αβ KOSF1). Although these animals exhibited no significant changes in the development of the ventromedial hypothalamus, we noted pronounced hypotrophy in the adrenal cortex, testis, and ovary. Additionally, corticosterone and aldosterone levels were significantly reduced. The absence of these subunits also resulted in decreased body weight and survival rate, along with impaired glucose homeostasis, in p110αβ KOSF1 mice.
Conclusion
The data demonstrate the specific roles of PI3K catalytic subunits in the development and function of SF1-expressing organs.
Figure
Reference
-
1. Morohashi KI, Inoue M, Baba T. Coordination of multiple cellular processes by NR5A1/Nr5a1. Endocrinol Metab (Seoul). 2020; 35:756–64.
Article2. Parker KL, Ikeda Y, Luo X. The roles of steroidogenic factor-1 in reproductive function. Steroids. 1996; 61:161–5.
Article3. Ikeda Y, Luo X, Abbud R, Nilson JH, Parker KL. The nuclear receptor steroidogenic factor 1 is essential for the formation of the ventromedial hypothalamic nucleus. Mol Endocrinol. 1995; 9:478–86.
Article4. Luo X, Ikeda Y, Parker KL. A cell-specific nuclear receptor is essential for adrenal and gonadal development and sexual differentiation. Cell. 1994; 77:481–90.
Article5. Fujikawa T, Choi YH, Yang DJ, Shin DM, Donato J Jr, Kohno D, et al. P110β in the ventromedial hypothalamus regulates glucose and energy metabolism. Exp Mol Med. 2019; 51:1–9.
Article6. Kim KW, Sohn JW, Kohno D, Xu Y, Williams K, Elmquist JK. SF1 in the ventral medial hypothalamic nucleus: a key regulator of homeostasis. Mol Cell Endocrinol. 2011; 336:219–23.
Article7. Xu Y, Hill JW, Fukuda M, Gautron L, Sohn JW, Kim KW, et al. PI3K signaling in the ventromedial hypothalamic nucleus is required for normal energy homeostasis. Cell Metab. 2010; 12:88–95.
Article8. Alam H, Maizels ET, Park Y, Ghaey S, Feiger ZJ, Chandel NS, et al. Follicle-stimulating hormone activation of hypoxia-inducible factor-1 by the phosphatidylinositol 3-kinase/AKT/Ras homolog enriched in brain (Rheb)/mammalian target of rapamycin (mTOR) pathway is necessary for induction of select protein markers of follicular differentiation. J Biol Chem. 2004; 279:19431–40.
Article9. Jean S, Kiger AA. Classes of phosphoinositide 3-kinases at a glance. J Cell Sci. 2014; 127(Pt 5):923–8.
Article10. Vanhaesebroeck B, Guillermet-Guibert J, Graupera M, Bilanges B. The emerging mechanisms of isoform-specific PI3K signalling. Nat Rev Mol Cell Biol. 2010; 11:329–41.
Article11. Shepherd PR, Withers DJ, Siddle K. Phosphoinositide 3-kinase: the key switch mechanism in insulin signalling. Biochem J. 1998; 333(Pt 3):471–90.
Article12. Wymann MP, Pirola L. Structure and function of phosphoinositide 3-kinases. Biochim Biophys Acta. 1998; 1436:127–50.
Article13. Carpenter CL, Cantley LC. Phosphoinositide 3-kinase and the regulation of cell growth. Biochim Biophys Acta. 1996; 1288:M11–6.
Article14. Ciraolo E, Morello F, Hobbs RM, Wolf F, Marone R, Iezzi M, et al. Essential role of the p110beta subunit of phosphoinositide 3-OH kinase in male fertility. Mol Biol Cell. 2010; 21:704–11.15. Reddy P, Liu L, Adhikari D, Jagarlamudi K, Rajareddy S, Shen Y, et al. Oocyte-specific deletion of Pten causes premature activation of the primordial follicle pool. Science. 2008; 319:611–3.
Article16. Su H, Gu Y, Li F, Wang Q, Huang B, Jin X, et al. The PI3K/AKT/mTOR signaling pathway is overactivated in primary aldosteronism. PLoS One. 2013; 8:e62399.
Article17. Tevosian SG, Fox SC, Ghayee HK. Molecular mechanisms of primary aldosteronism. Endocrinol Metab (Seoul). 2019; 34:355–66.
Article18. Saito K, He Y, Yang Y, Zhu L, Wang C, Xu P, et al. PI3K in the ventromedial hypothalamic nucleus mediates estrogenic actions on energy expenditure in female mice. Sci Rep. 2016; 6:23459.
Article19. Sohn JW, Oh Y, Kim KW, Lee S, Williams KW, Elmquist JK. Leptin and insulin engage specific PI3K subunits in hypothalamic SF1 neurons. Mol Metab. 2016; 5:669–79.
Article20. Vanhaesebroeck B, Leevers SJ, Panayotou G, Waterfield MD. Phosphoinositide 3-kinases: a conserved family of signal transducers. Trends Biochem Sci. 1997; 22:267–72.
Article21. Dhillon H, Zigman JM, Ye C, Lee CE, McGovern RA, Tang V, et al. Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron. 2006; 49:191–203.
Article22. Jia S, Liu Z, Zhang S, Liu P, Zhang L, Lee SH, et al. Essential roles of PI(3)K-p110beta in cell growth, metabolism and tumorigenesis. Nature. 2008; 454:776–9.
Article23. Zhao JJ, Cheng H, Jia S, Wang L, Gjoerup OV, Mikami A, et al. The p110alpha isoform of PI3K is essential for proper growth factor signaling and oncogenic transformation. Proc Natl Acad Sci U S A. 2006; 103:16296–300.24. Kim KW, Zhao L, Donato J Jr, Kohno D, Xu Y, Elias CF, et al. Steroidogenic factor 1 directs programs regulating dietinduced thermogenesis and leptin action in the ventral medial hypothalamic nucleus. Proc Natl Acad Sci U S A. 2011; 108:10673–8.
Article25. Tran LT, Park S, Kim SK, Lee JS, Kim KW, Kwon O. Hypothalamic control of energy expenditure and thermogenesis. Exp Mol Med. 2022; 54:358–69.
Article26. Sun JS, Yang DJ, Kinyua AW, Yoon SG, Seong JK, Kim J, et al. Ventromedial hypothalamic primary cilia control energy and skeletal homeostasis. J Clin Invest. 2021; 131:e138107.
Article27. Tartaglia LA, Dembski M, Weng X, Deng N, Culpepper J, Devos R, et al. Identification and expression cloning of a leptin receptor, OB-R. Cell. 1995; 83:1263–71.
Article28. Yokosuka M, Okamura H, Hayashi S. Postnatal development and sex difference in neurons containing estrogen receptor-alpha immunoreactivity in the preoptic brain, the diencephalon, and the amygdala in the rat. J Comp Neurol. 1997; 389:81–93.
Article29. Kia HK, Krebs CJ, Koibuchi N, Chin WW, Pfaff DW. Coexpression of estrogen and thyroid hormone receptors in individual hypothalamic neurons. J Comp Neurol. 2001; 437:286–95.
Article30. Parker KL, Schimmer BP. The role of nuclear receptors in steroid hormone production. Semin Cancer Biol. 1994; 5:317–25.31. Nagatsu T, Levitt M, Udenfriend S. Tyrosine hydroxylase. the initial step in norepinephrine biosynthesis. J Biol Chem. 1964; 239:2910–7.32. Petrack B, Sheppy F, Fetzer V. Studies on tyrosine hydroxylase from bovine adrenal medulla. J Biol Chem. 1968; 243:743–8.
Article33. Shen WH, Moore CC, Ikeda Y, Parker KL, Ingraham HA. Nuclear receptor steroidogenic factor 1 regulates the müllerian inhibiting substance gene: a link to the sex determination cascade. Cell. 1994; 77:651–61.
Article34. Clemens JW, Lala DS, Parker KL, Richards JS. Steroidogenic factor-1 binding and transcriptional activity of the cholesterol side-chain cleavage promoter in rat granulosa cells. Endocrinology. 1994; 134:1499–508.
Article35. Turathum B, Gao EM, Chian RC. The function of cumulus cells in oocyte growth and maturation and in subsequent ovulation and fertilization. Cells. 2021; 10:2292.
Article36. Khanh DV, Choi YH, Moh SH, Kinyua AW, Kim KW. Leptin and insulin signaling in dopaminergic neurons: relationship between energy balance and reward system. Front Psychol. 2014; 5:846.
Article37. Klockener T, Hess S, Belgardt BF, Paeger L, Verhagen LA, Husch A, et al. High-fat feeding promotes obesity via insulin receptor/PI3K-dependent inhibition of SF1 VMH neurons. Nat Neurosci. 2011; 14:911–8.
Article38. Renshaw J, Taylor KR, Bishop R, Valenti M, De Haven Brandon A, Gowan S, et al. Dual blockade of the PI3K/AKT/mTOR (AZD8055) and RAS/MEK/ERK (AZD6244) pathways synergistically inhibits rhabdomyosarcoma cell growth in vitro and in vivo. Clin Cancer Res. 2013; 19:5940–51.39. Ng SS, Tsao MS, Chow S, Hedley DW. Inhibition of phosphatidylinositide 3-kinase enhances gemcitabine-induced apoptosis in human pancreatic cancer cells. Cancer Res. 2000; 60:5451–5.40. Matheny RW Jr, Adamo ML. PI3K p110 alpha and p110 beta have differential effects on Akt activation and protection against oxidative stress-induced apoptosis in myoblasts. Cell Death Differ. 2010; 17:677–88.
Article41. Hatano O, Takayama K, Imai T, Waterman MR, Takakusu A, Omura T, et al. Sex-dependent expression of a transcription factor, Ad4BP, regulating steroidogenic P-450 genes in the gonads during prenatal and postnatal rat development. Development. 1994; 120:2787–97.
Article42. Huang CC, Miyagawa S, Matsumaru D, Parker KL, Yao HH. Progenitor cell expansion and organ size of mouse adrenal is regulated by sonic hedgehog. Endocrinology. 2010; 151:1119–28.
Article43. Morohashi K. The ontogenesis of the steroidogenic tissues. Genes Cells. 1997; 2:95–106.
Article44. Mesiano S, Jaffe RB. Developmental and functional biology of the primate fetal adrenal cortex. Endocr Rev. 1997; 18:378–403.
Article45. Ikeda Y, Shen WH, Ingraham HA, Parker KL. Developmental expression of mouse steroidogenic factor-1, an essential regulator of the steroid hydroxylases. Mol Endocrinol. 1994; 8:654–62.
Article46. Ikeda Y, Lala DS, Luo X, Kim E, Moisan MP, Parker KL. Characterization of the mouse FTZ-F1 gene, which encodes a key regulator of steroid hydroxylase gene expression. Mol Endocrinol. 1993; 7:852–60.
Article47. Morohashi K, Hatano O, Nomura M, Takayama K, Hara M, Yoshii H, et al. Function and distribution of a steroidogenic cell-specific transcription factor, Ad4BP. J Steroid Biochem Mol Biol. 1995; 53:81–8.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Role of the PI3K Pathway in the Regeneration of the Damaged Brain by Neural Stem Cells after Cerebral Infarction
- Tumor Cell Clone Expressing the Membrane-bound Form of IL-12p35 Subunit Stimulates Antitumor Immune Responses Dominated by CD8+ T Cells
- MSCs-Derived miR-150-5p-Expressing Exosomes Promote Skin Wound Healing by Activating PI3K/AKT Pathway through PTEN
- Effect of Developmental Lead Exposure on the Expression of Hippocampal NMDA Receptor Subunit mRNA
- 7alpha-Hydroxycholesterol Elicits TLR6-Mediated Expression of IL-23 in Monocytic Cells