Cancer Res Treat.
2024 Oct;56(4):1262-1269. 10.4143/crt.2024.047.
Long-term Psychiatric and Endocrine Complications Following Hematopoietic Stem Cell Transplantation in Hematologic Disease in Korea: A Nation-Wide Cohort Study
- Affiliations
-
- 1Division of Hemato-Oncology, Department of Internal Medicine, Korea University Guro Hospital, Korea University College of Medicine, Seoul, Korea
- 2Smart Healthcare Center, Korea University Guro Hospital, Korea University College of Medicine, Seoul, Korea
- KMID: 2560259
- DOI: http://doi.org/10.4143/crt.2024.047
Abstract
- Purpose
Numerous patients experience long-term complications after hematopoietic stem cell transplantation (HSCT). This study aimed to identify the frequency and risk factors for psychiatric and endocrine complications following HSCT through big data analyses.
Materials and Methods
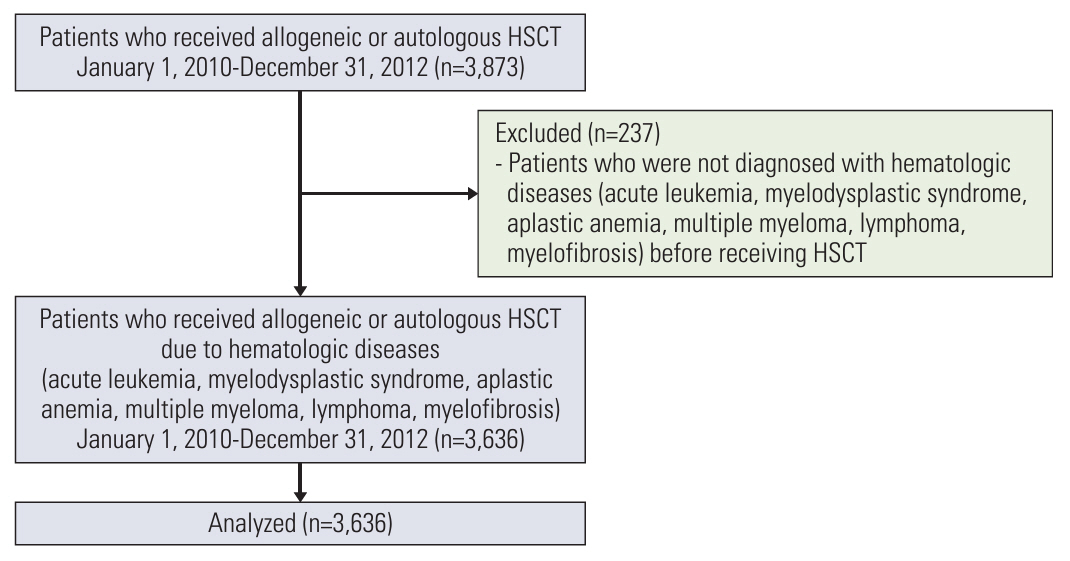
We established a cohort of patients with hematologic disease who underwent HSCT in Korea between 2010 and 2012 using the Health Insurance Review & Assessment Service data. A total of 3,636 patients were identified, and insurance claims were tracked using psychiatric and endocrine diagnostic International Classification of Diseases, 10th Revision codes for the ensuing decade. We identified the incidence rates of long-term complications based on the baseline disease and HSCT type. Prognostic factors for each complication were scrutinized using logistic regression analysis.
Results
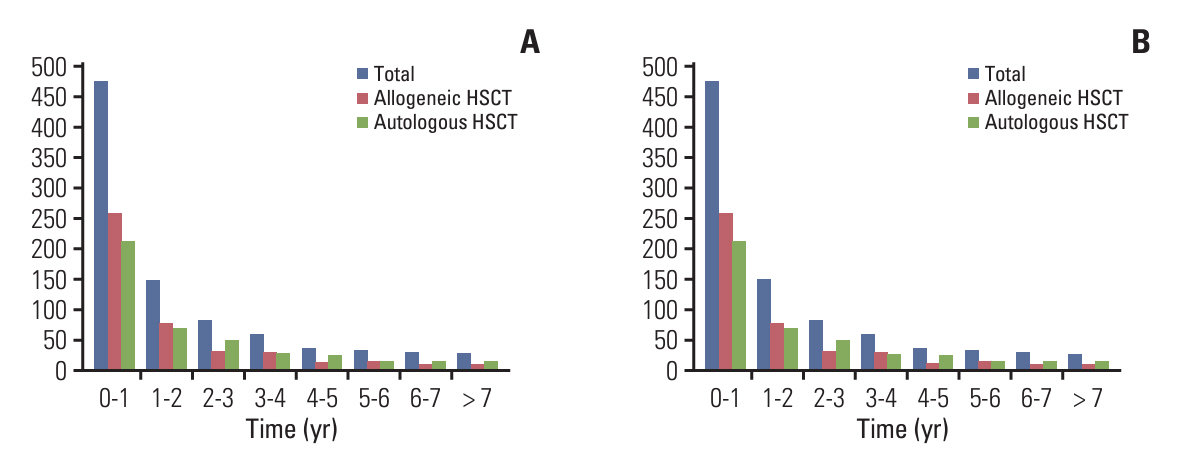
A total of 1,879 patients underwent allogeneic HSCT and 1,757 patients received autologous HSCT. Post-HSCT, 506 patients were diagnosed with depression, 465 with anxiety disorders, and 659 with diabetes. The highest incidence of long-term complications occurred within the first year post-HSCT (12.2%), subsequently decreasing over time. Risk factors for depressive disorders after allogeneic HSCT included female sex, a total body irradiation–based conditioning regimen, and cyclosporine. Identified risk factors for diabetes mellitus comprised old age, total body irradiation–based conditioning regimen, and non-antithymocyte globulin protocol. Regarding autologous HSCT, only female sex was identified as a risk factor for depressive disorders, whereas elderly patients and those with multiple myeloma were identified as poor prognostic factors for diabetes mellitus.
Conclusion
The incidence of long-term psychiatric and endocrine complications post-HSCT remains high, and patients with risk factors for these complications require vigilant follow-up.
Keyword
Figure
Reference
-
References
1. Majhail NS, Farnia SH, Carpenter PA, Champlin RE, Crawford S, Marks DI, et al. Indications for autologous and allogeneic hematopoietic cell transplantation: guidelines from the American Society for Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2015; 21:1863–9.2. Passweg JR, Baldomero H, Chabannon C, Basak GW, de la Camara R, Corbacioglu S, et al. Hematopoietic cell transplantation and cellular therapy survey of the EBMT: monitoring of activities and trends over 30 years. Bone Marrow Transplant. 2021; 56:1651–64.
Article3. D’Souza A, Fretham C, Lee SJ, Arora M, Brunner J, Chhabra S, et al. Current use of and trends in hematopoietic cell transplantation in the United States. Biol Blood Marrow Transplant. 2020; 26:e177–82.4. Iida M, Kodera Y, Dodds A, Ho AYL, Nivison-Smith I, Akter MR, et al. Advances in hematopoietic stem cell transplantation in the Asia-Pacific region: the second report from APBMT 2005-2015. Bone Marrow Transplant. 2019; 54:1973–86.
Article5. Maurer K, Ho VT, Inyang E, Cutler C, Koreth J, Shapiro RM, et al. Posttransplant cyclophosphamide vs tacrolimus-based GVHD prophylaxis: lower incidence of relapse and chronic GVHD. Blood Adv. 2023; 7:3903–15.
Article6. Niederwieser D, Baldomero H, Bazuaye N, Bupp C, Chaudhri N, Corbacioglu S, et al. One and a half million hematopoietic stem cell transplants: continuous and differential improvement in worldwide access with the use of non-identical family donors. Haematologica. 2022; 107:1045–53.
Article7. Gagelmann N, Kroger N. Dose intensity for conditioning in allogeneic hematopoietic cell transplantation: can we recommend “when and for whom” in 2021? Haematologica. 2021; 106:1794–804.
Article8. Muffly L, Pasquini MC, Martens M, Brazauskas R, Zhu X, Adekola K, et al. Increasing use of allogeneic hematopoietic cell transplantation in patients aged 70 years and older in the United States. Blood. 2017; 130:1156–64.
Article9. Passweg JR, Baldomero H, Bader P, Bonini C, Duarte RF, Dufour C, et al. Use of haploidentical stem cell transplantation continues to increase: the 2015 European Society for Blood and Marrow Transplant activity survey report. Bone Marrow Transplant. 2017; 52:811–7.
Article10. Wauben B, van Yperen NC, van der Poel MW, Kohler S, van Greevenbroek MM, Schouten HC. Assessing long-term effects after stem cell transplantation: design of the MOSA study. J Clin Epidemiol. 2022; 148:10–6.
Article11. Gratwohl A, Brand R, Frassoni F, Rocha V, Niederwieser D, Reusser P, et al. Cause of death after allogeneic haematopoietic stem cell transplantation (HSCT) in early leukaemias: an EBMT analysis of lethal infectious complications and changes over calendar time. Bone Marrow Transplant. 2005; 36:757–69.
Article12. Majhail NS. Long-term complications after hematopoietic cell transplantation. Hematol Oncol Stem Cell Ther. 2017; 10:220–7.
Article13. Wingard JR, Majhail NS, Brazauskas R, Wang Z, Sobocinski KA, Jacobsohn D, et al. Long-term survival and late deaths after allogeneic hematopoietic cell transplantation. J Clin Oncol. 2011; 29:2230–9.
Article14. Martin PJ, Counts GW Jr, Appelbaum FR, Lee SJ, Sanders JE, Deeg HJ, et al. Life expectancy in patients surviving more than 5 years after hematopoietic cell transplantation. J Clin Oncol. 2010; 28:1011–6.
Article15. Kim GE, Jo MW, Shin YW. Increased prevalence of depression in South Korea from 2002 to 2013. Sci Rep. 2020; 10:16979.
Article16. Hjermstad MJ, Loge JH, Evensen SA, Kvaloy SO, Fayers PM, Kaasa S. The course of anxiety and depression during the first year after allogeneic or autologous stem cell transplantation. Bone Marrow Transplant. 1999; 24:1219–28.
Article17. Cheon J, Lee YJ, Jo JC, Kweon K, Koh S, Min YJ, et al. Late complications and quality of life assessment for survivors receiving allogeneic hematopoietic stem cell transplantation. Support Care Cancer. 2021; 29:975–86.
Article18. Rueda-Lara M, Lopez-Patton MR. Psychiatric and psychosocial challenges in patients undergoing haematopoietic stem cell transplants. Int Rev Psychiatry. 2014; 26:74–86.
Article19. Sun CL, Francisco L, Baker KS, Weisdorf DJ, Forman SJ, Bhatia S. Adverse psychological outcomes in long-term survivors of hematopoietic cell transplantation: a report from the Bone Marrow Transplant Survivor Study (BMTSS). Blood. 2011; 118:4723–31.
Article20. Nakamura ZM, Nash RP, Quillen LJ, Richardson DR, McCall RC, Park EM. Psychiatric care in hematopoietic stem cell transplantation. Psychosomatics. 2019; 60:227–37.
Article21. Hirabayashi K, Nakazawa Y, Matsuura H, Hara Y, Kurata T, Hirabayashi K, et al. Risk factors for diabetes mellitus and impaired glucose tolerance following allogeneic hematopoietic stem cell transplantation in pediatric patients with hematological malignancies. Int J Hematol. 2014; 99:477–86.
Article22. Atilla E, Atilla PA, Toprak SK, Demirer T. A review of late complications of allogeneic hematopoietic stem cell transplantations. Clin Transplant. 2017; 31:e13062.
Article23. Chemaitilly W, Sklar CA. Endocrine complications of hematopoietic stem cell transplantation. Endocrinol Metab Clin North Am. 2007; 36:983–98.
Article24. Griffith ML, Jagasia M, Jagasia SM. Diabetes mellitus after hematopoietic stem cell transplantation. Endocr Pract. 2010; 16:699–706.
Article25. Baker KS, Ness KK, Steinberger J, Carter A, Francisco L, Burns LJ, et al. Diabetes, hypertension, and cardiovascular events in survivors of hematopoietic cell transplantation: a report from the bone marrow transplantation survivor study. Blood. 2007; 109:1765–72.
Article26. Neville KA, Cohn RJ, Steinbeck KS, Johnston K, Walker JL. Hyperinsulinemia, impaired glucose tolerance, and diabetes mellitus in survivors of childhood cancer: prevalence and risk factors. J Clin Endocrinol Metab. 2006; 91:4401–7.
Article27. Bonanomi S, Gaiero A, Masera N, Rovelli A, Uderzo C, Fichera G, et al. Distinctive characteristics of diabetes mellitus after hematopoietic cell transplantation during childhood. Pediatr Transplant. 2006; 10:461–5.
Article28. Subramanian S, Trence DL. Immunosuppressive agents: effects on glucose and lipid metabolism. Endocrinol Metab Clin North Am. 2007; 36:891–905.29. Penfornis A, Kury-Paulin S. Immunosuppressive drug-induced diabetes. Diabetes Metab. 2006; 32:539–46.
Article30. Ravindran VK, Moore RH, Dunseath G, Luzio SD, Owens DR, Baboolal K. Insulin hyposecretion in nondiabetic, tacrolimus-treated renal transplant recipients more than 6 months posttransplantation. Transplantation. 2009; 87:1870–6.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Hematopoietic stem cell transplantation: overview for general pediatrician
- Opening the era of in vivo xenotransplantation model for hematopoietic stem cell transplantation
- Comparison of Quality of Life of Autologous and Allogeneic Hematopoietic Stem Cell Transplantation Recipients
- A Study of the Factors Affecting the Term of Engraftment During Hematopoietic Stem Cell Transplantation with a Focus on the Inhibitors of Oral Intake and the Period of Nutritional Support
- Hematopoietic Stem Cell Transplantation