Cancer Res Treat.
2024 Oct;56(4):1252-1261. 10.4143/crt.2024.104.
Stratified Treatment in Pediatric Anaplastic Large Cell Lymphoma: Result of a Prospective Open-Label Multiple-Institution Study
- Affiliations
-
- 1Department of Pediatric Oncology, Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in Southern China, and Collaborative Innovation Center of Cancer Medicine, Guangzhou, China
- 2The First Affiliated Hospital of Guangxi Medical University, Nanning, China
- 3Guangzhou Women and Children's Medical Center, Guangzhou Medical University, Guangzhou, China
- 4Union Hospital Affiliated to Fujian Medical University, Fuzhou, China
- 5Shenzhen Children’s Hospital, Shenzhen, China
- 6Kunming Children's Hospital, Kunming, China
- 7Nanfang Hospital Southern Medical University, Guangzhou, China
- 8Xiangya Hospital Central South University, Changsha, China
- 9The Second Xiangya Hospital Central South University, Hunan, China
- 10The Affiliated Cancer Hospital of Zhengzhou University, Henan Cancer Hospital, Zhengzhou, China
- 11Sun Yat-Sen Memorial Hospital, Sun Yat-Sen University, Guangzhou, China
- 12The First Affiliated Hospital of Sun Yat-Sen University, Guangzhou, China
- 13The First Affiliated Hospital of Guangzhou Medical University, Guangzhou, China
- 14ZhuJiang Hospital Southern Medical University, Guangzhou, China
- 15Huizhou Municipal Central Hospital, Huizhou Central People's Hospital, Huizhou, China
- 16Hunan Children's Hospital, Changsha, China
- 17Hunan Provincial People's Hospital, First Affiliated Hospital of Hunan Normal University, Changsha, China
- KMID: 2560258
- DOI: http://doi.org/10.4143/crt.2024.104
Abstract
- Purpose
The risk stratification of pediatric anaplastic large cell lymphoma (ALCL) has not been standardized. In this study, new risk factors were included to establish a new risk stratification system for ALCL, and its feasibility in clinical practice was explored.
Materials and Methods
On the basis of the non-Hodgkin’s lymphoma Berlin–Frankfurt–Munster 95 (NHL-BFM-95) protocol, patients with minimal disseminated disease (MDD), high-risk tumor site (multiple bone, skin, liver, and lung involvement), and small cell/lymphohistiocytic (SC/LH) pathological subtype were enrolled in risk stratification. Patients were treated with a modified NHL-BFM-95 protocol combined with an anaplastic lymphoma kinase inhibitor or vinblastine (VBL).
Results
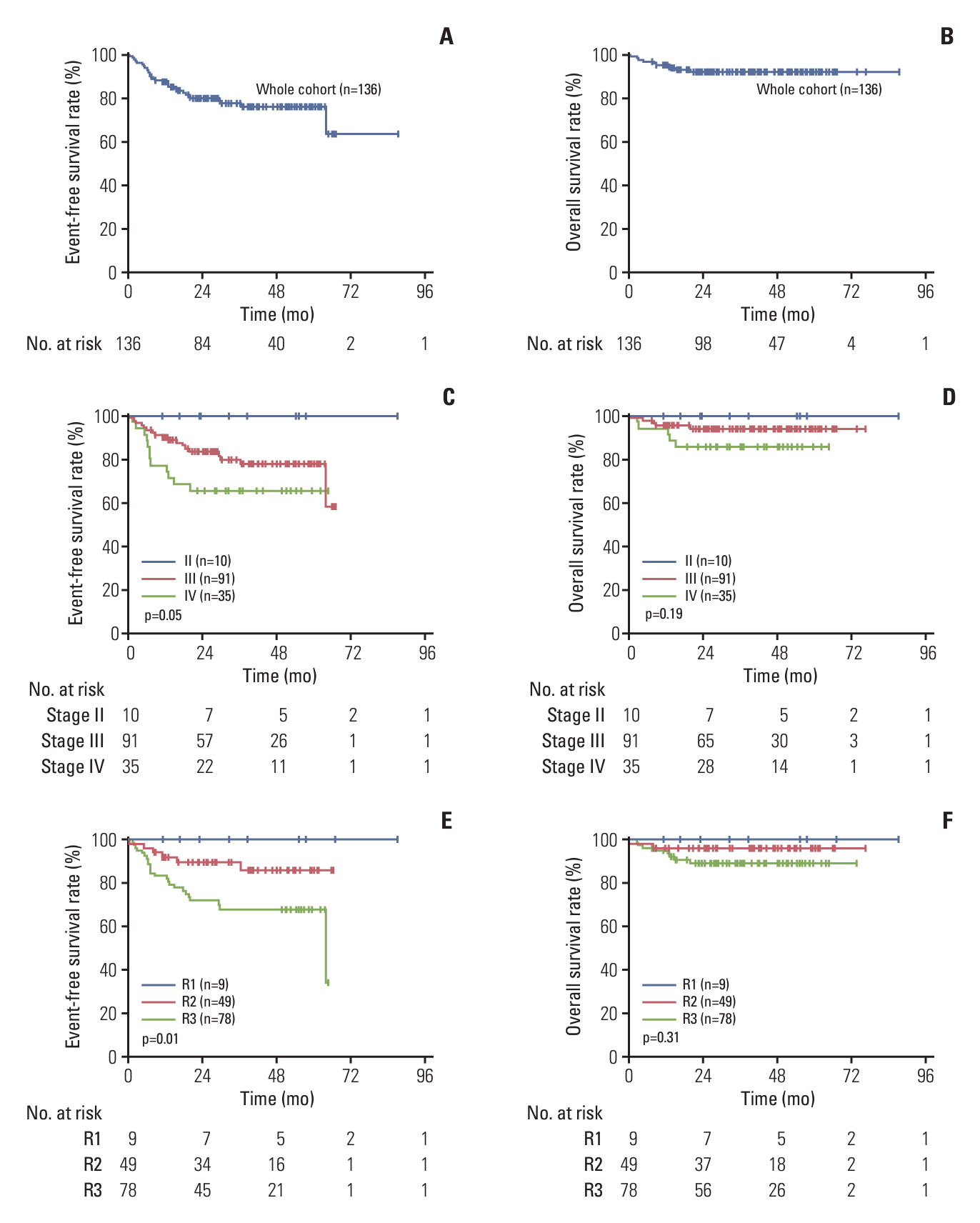
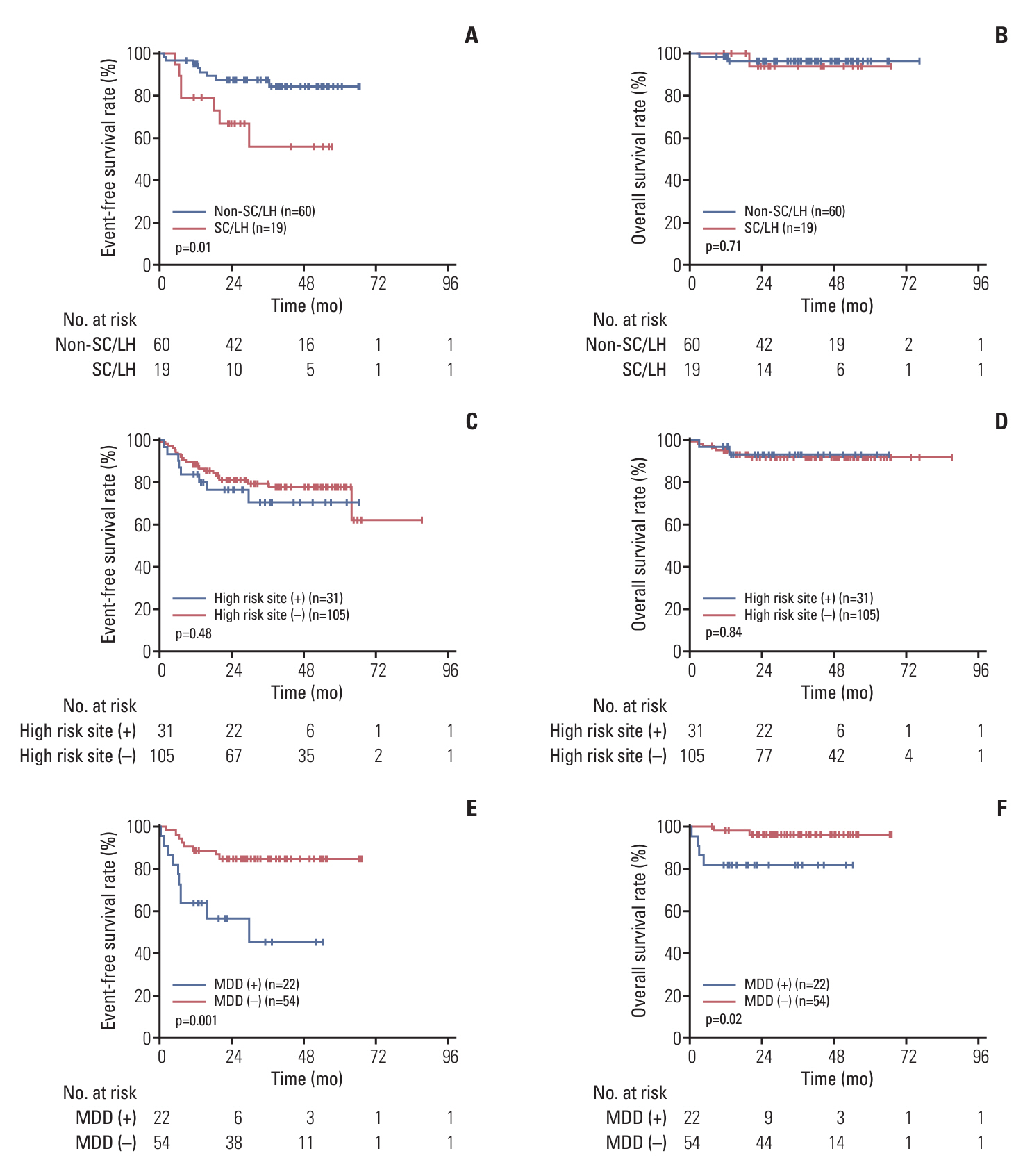
A total of 136 patients were enrolled in this study. The median age was 8.8 years. The 3-year event-free survival (EFS) and overall survival of the entire cohort were 77.7% (95% confidence interval [CI], 69.0% to 83.9%) and 92.3% (95% CI, 86.1% to 95.8%), respectively. The 3-year EFS rates of low-risk group (R1), intermediate-risk group (R2), and high-risk group (R3) patients were 100%, 89.5% (95% CI, 76.5% to 95.5%), and 67.9% (95% CI, 55.4% to 77.6%), respectively. The prognosis of patients with MDD (+), stage IV cancer, SC/LH lymphoma, and high-risk sites was poor, and the 3-year EFS rates were 45.3% (95% CI, 68.6% to 19.0%), 65.7% (95% CI, 47.6% to 78.9%), 55.7% (95% CI, 26.2% to 77.5%), and 70.7% (95% CI, 48.6% to 84.6%), respectively. At the end of follow-up, one of the five patients who received maintenance therapy with VBL relapsed, and seven patients receiving anaplastic lymphoma kinase inhibitor maintenance therapy did not experience relapse.
Conclusion
This study has confirmed the poor prognostic of MDD (+), high-risk site and SC/LH, but patients with SC/LH lymphoma and MDD (+) at diagnosis still need to receive better treatment (ClinicalTrials.gov number, NCT03971305).
Keyword
Figure
Reference
-
References
1. Lowe EJ, Gross TG. Anaplastic large cell lymphoma in children and adolescents. Pediatr Hematol Oncol. 2013; 30:509–19.
Article2. Sandlund JT, Downing JR, Crist WM. Non-Hodgkin’s lymphoma in childhood. N Engl J Med. 1996; 334:1238–48.
Article3. Seidemann K, Tiemann M, Schrappe M, Yakisan E, Simonitsch I, Janka-Schaub G, et al. Short-pulse B-non-Hodgkin lymphoma-type chemotherapy is efficacious treatment for pediatric anaplastic large cell lymphoma: a report of the Berlin-Frankfurt-Munster Group Trial NHL-BFM 90. Blood. 2001; 97:3699–706.
Article4. Yi SH, Zou DH, Ken LG. Decipher the 2016 revision of the World Health Organization classification of lymphoid neoplasms. Zhonghua Yi Xue Za Zhi. 2016; 96:3365–9.5. Lamant L, McCarthy K, d’Amore E, Klapper W, Nakagawa A, Fraga M, et al. Prognostic impact of morphologic and phenotypic features of childhood ALK-positive anaplastic large-cell lymphoma: results of the ALCL99 study. J Clin Oncol. 2011; 29:4669–76.
Article6. Furqan F, Ahn KW, Chen Y, Kaur M, Abutalib SA, Ahmed N, et al. Allogeneic haematopoietic cell transplant in patients with relapsed/refractory anaplastic large cell lymphoma. Br J Haematol. 2023; 200:54–63.
Article7. Knorr F, Brugieres L, Pillon M, Zimmermann M, Ruf S, Attarbaschi A, et al. Stem cell transplantation and vinblastine monotherapy for relapsed pediatric anaplastic large cell lymphoma: results of the international, prospective ALCL-relapse trial. J Clin Oncol. 2020; 38:3999–4009.
Article8. Woessmann W, Zimmermann M, Lenhard M, Burkhardt B, Rossig C, Kremens B, et al. Relapsed or refractory anaplastic large-cell lymphoma in children and adolescents after BerlinFrankfurt-Muenster (BFM)-type first-line therapy: a BFMgroup study. J Clin Oncol. 2011; 29:3065–71.
Article9. Mussolin L, Le Deley MC, Carraro E, Damm-Welk C, Attarbaschi A, Williams D, et al. Prognostic factors in childhood anaplastic large cell lymphoma: long term results of the international ALCL99 trial. Cancers (Basel). 2020; 12:2747.
Article10. Damm-Welk C, Mussolin L, Zimmermann M, Pillon M, Klapper W, Oschlies I, et al. Early assessment of minimal residual disease identifies patients at very high relapse risk in NPM-ALK-positive anaplastic large-cell lymphoma. Blood. 2014; 123:334–7.
Article11. Mussolin L, Pillon M, d’Amore ES, Santoro N, Lombardi A, Fagioli F, et al. Prevalence and clinical implications of bone marrow involvement in pediatric anaplastic large cell lymphoma. Leukemia. 2005; 19:1643–7.
Article12. Damm-Welk C, Busch K, Burkhardt B, Schieferstein J, Viehmann S, Oschlies I, et al. Prognostic significance of circulating tumor cells in bone marrow or peripheral blood as detected by qualitative and quantitative PCR in pediatric NPM-ALK-positive anaplastic large-cell lymphoma. Blood. 2007; 110:670–7.
Article13. Le Deley MC, Reiter A, Williams D, Delsol G, Oschlies I, McCarthy K, et al. Prognostic factors in childhood anaplastic large cell lymphoma: results of a large European intergroup study. Blood. 2008; 111:1560–6.
Article14. Yang HB, Li J, Shen T. Primary anaplastic large cell lymphoma of the lung: report of two cases and literature review. Acta Haematol. 2007; 118:188–91.15. Murphy SB. Classification, staging and end results of treatment of childhood non-Hodgkin’s lymphomas: dissimilarities from lymphomas in adults. Semin Oncol. 1980; 7:332–9.16. Brugieres L, Le Deley MC, Rosolen A, Williams D, Horibe K, Wrobel G, et al. Impact of the methotrexate administration dose on the need for intrathecal treatment in children and adolescents with anaplastic large-cell lymphoma: results of a randomized trial of the EICNHL Group. J Clin Oncol. 2009; 27:897–903.
Article17. Lowe EJ, Sposto R, Perkins SL, Gross TG, Finlay J, Zwick D, et al. Intensive chemotherapy for systemic anaplastic large cell lymphoma in children and adolescents: final results of Children’s Cancer Group Study 5941. Pediatr Blood Cancer. 2009; 52:335–9.
Article18. Ram-Wolff C, Vercellino L, Brice P, La Selva R, Bagot M. (18) F-fluorodeoxyglucose-positron emission tomography is more sensitive than computed tomography in initial staging of patients with an anaplastic T-cell lymphoma first presenting in the skin. Eur J Dermatol. 2017; 27:496–504.
Article19. Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014; 32:3059–68.
Article20. Johnson SA, Kumar A, Matasar MJ, Schoder H, Rademaker J. Imaging for staging and response assessment in lymphoma. Radiology. 2015; 276:323–38.
Article21. Mosse YP, Voss SD, Lim MS, Rolland D, Minard CG, Fox E, et al. Targeting ALK with crizotinib in pediatric anaplastic large cell lymphoma and inflammatory myofibroblastic tumor: a Children’s Oncology Group Study. J Clin Oncol. 2017; 35:3215–21.
Article22. Mediwake H, Morris K, Curley C, Butler J, Kennedy G. Use of brentuximab vedotin as salvage therapy pre-allogeneic stem cell transplantation in relapsed/refractory CD30 positive lympho-proliferative disorders: a single centre experience. Intern Med J. 2017; 47:574–8.23. Lowe EJ, Reilly AF, Lim MS, Gross TG, Saguilig L, Barkauskas DA, et al. Brentuximab vedotin in combination with chemotherapy for pediatric patients with ALK+ ALCL: results of COG trial ANHL12P1. Blood. 2021; 137:3595–603.
Article24. Lowe EJ, Reilly AF, Lim MS, Gross TG, Saguilig L, Barkauskas DA, et al. Crizotinib in combination with chemotherapy for pediatric patients with ALK+ anaplastic large-cell lymphoma: the results of Children’s Oncology Group Trial ANHL12P1. J Clin Oncol. 2023; 41:2043–53.
Article25. Le Deley MC, Rosolen A, Williams DM, Horibe K, Wrobel G, Attarbaschi A, et al. Vinblastine in children and adolescents with high-risk anaplastic large-cell lymphoma: results of the randomized ALCL99-vinblastine trial. J Clin Oncol. 2010; 28:3987–93.
Article26. Chen SH, Chen JS, Jou ST, Wu KH, Hung IJ, Sheen JM, et al. Outcome and prognosis of anaplastic large cell lymphoma in children: a report from the Taiwan Pediatric Oncology Group. Leuk Lymphoma. 2019; 60:1942–9.
Article27. Mussolin L, Damm-Welk C, Pillon M, Zimmermann M, Franceschetto G, Pulford K, et al. Use of minimal disseminated disease and immunity to NPM-ALK antigen to stratify ALK-positive ALCL patients with different prognosis. Leukemia. 2013; 27:416–22.
Article28. Iijima-Yamashita Y, Mori T, Nakazawa A, Fukano R, Takimoto T, Tsurusawa M, et al. Prognostic impact of minimal disseminated disease and immune response to NPM-ALK in Japanese children with ALK-positive anaplastic large cell lymphoma. Int J Hematol. 2018; 107:244–50.
Article29. Damm-Welk C, Lovisa F, Contarini G, Ludersen J, Carraro E, Knorr F, et al. Quantification of minimal disease by digital PCR in ALK-positive anaplastic large cell lymphoma: a step towards risk stratification in international trials? Cancers (Basel). 2022; 14:1703.
Article30. Damm-Welk C, Kutscher N, Zimmermann M, Attarbaschi A, Schieferstein J, Knorr F, et al. Quantification of minimal disseminated disease by quantitative polymerase chain reaction and digital polymerase chain reaction for NPM-ALK as a prognostic factor in children with anaplastic large cell lymphoma. Haematologica. 2020; 105:2141–9.
Article31. Brink M, Meeuwes FO, van der Poel MW, Kersten MJ, Wondergem M, Mutsaers P, et al. Impact of etoposide and ASCT on survival among patients aged <65 years with stage II to IV PTCL: a population-based cohort study. Blood. 2022; 140:1009–19.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Dermatofibroma in Patient with Relapsing Primary Cutaneous Anaplastic Large Cell Lymphoma
- A Case of Multiple Cranial Neuropathies Caused by Anaplastic Lymphoma Kinase-Negative Anaplastic Large Cell Lymphoma
- CD30-Positive Anaplastic Lymphoma Kinase-Negative Systemic Anaplastic Large-Cell Lymphoma in a 9-Year-Old Boy
- A case of Ki-1 positive large-cell lymphoma
- Immunohistochemical Study for Ki-1 and EMA Antigens in Large Cell Lymphoma including Anaplastic Large Cell Lymphoma