Nutr Res Pract.
2024 Oct;18(5):633-646. 10.4162/nrp.2024.18.5.633.
Cladophora glomerata Kützing extract exhibits antioxidant, anti-inflammation, and anti-nitrosative stress against impairment of renal organic anion transport in an in vivo study
- Affiliations
-
- 1Division of Physiology, School of Medical Sciences, University of Phayao, Phayao 56000, Thailand
- 2Office of Educational Affairs, Faculty of Abhaibhubejhr Thai Traditional Medicine, Burapha University, Chon Buri 20131, Thailand
- 3Chakri Naruebodindra Medical Institute, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Samut Prakan 10540, Thailand
- 4Center of Excellence in Agricultural Innovation for Graduate Entrepreneurs and Faculty of Fisheries Technology and Aquatic Resources, Maejo University, Chiang Mai 50290, Thailand
- 5Innovative Research Unit of Epithelial Transport and Regulation (iETR), Department of Physiology, Faculty of Medicine, Chiang Mai University, Chiang Mai 50200, Thailand
- KMID: 2560049
- DOI: http://doi.org/10.4162/nrp.2024.18.5.633
Abstract
- BACKGROUND/OBJECTIVES
Cladophora glomerata extract (CGE), rich in polyphenols, was reported to exhibit antidiabetic and renoprotective effects by modulating the functions of protein kinases-mediated organic anion transporter 1 (Oat1) and 3 (Oat3) in rats with type 2 diabetes mellitus (T2DM). Nevertheless, the antioxidant effects of CGE on such renoprotection have not been investigated. This study examined the mechanisms involved in the antioxidant effects of CGE on renal organic anion transport function in an in vivo study.
MATERIALS/METHODS
Diabetes was induced in the rats through a high-fat diet combined with a single dose of 40 mg/kg body weight (BW) streptozotocin. Subsequently, normal-diet rats were supplemented with a vehicle or 1,000 mg/kg BW of CGE, while T2DM rats were supplemented with a vehicle, CGE, or 200 mg/kg BW of vitamin C for 12 weeks. The study evaluated the general characteristics of T2DM and renal oxidative stress markers. The renal organic transport function was assessed by measuring the para-aminohippurate (PAH) uptake using renal cortical slices and renal inflammatory cytokine expression in the normal diet (ND) and ND + CGE treated groups.
RESULTS
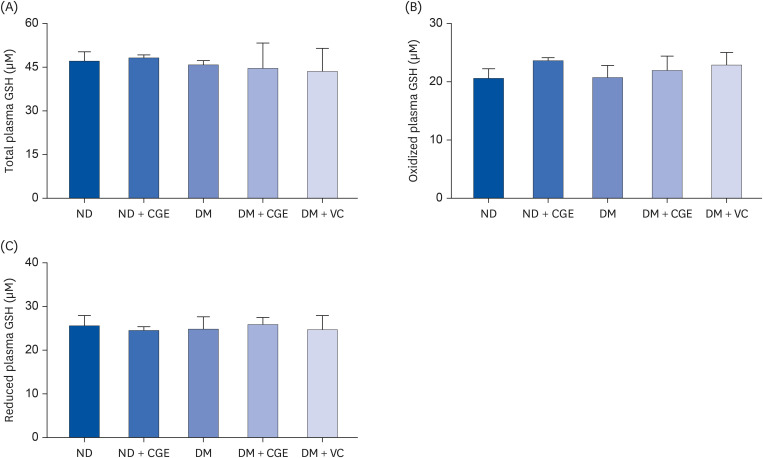
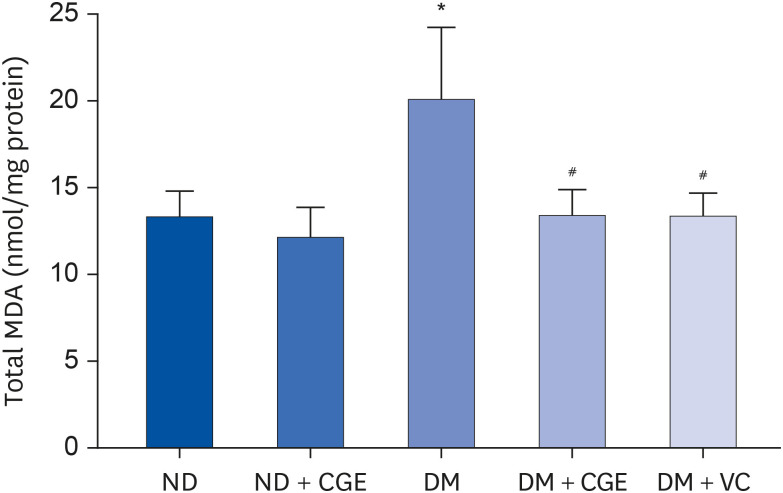
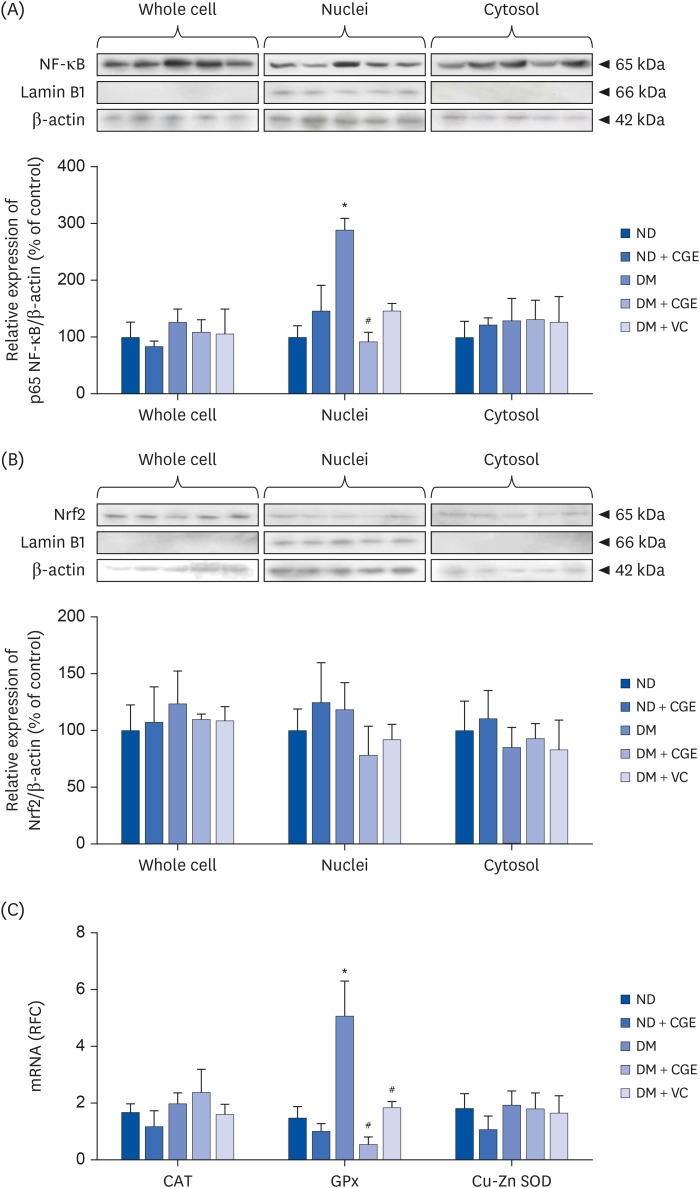
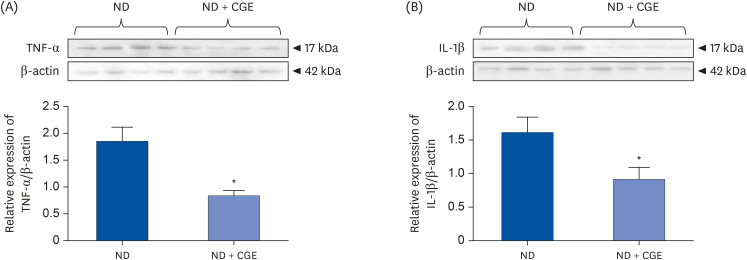
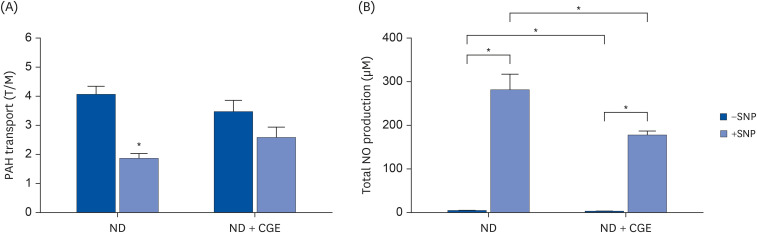
CGE supplementation significantly reduced hyperglycemia, hypertriglyceridemia, insulin resistance, and renal lipid peroxidation in T2DM rats. This was accompanied by the normalization of high expressions of renal glutathione peroxidase and nuclear factor kappa B by CGE and vitamin C. The renal anti-inflammation of CGE was evidenced by the reduction of tumor necrosis factor-1α and interleukin-1β. CGE directly blunted sodium nitroprusside-induced renal oxidative/nitrosative stresses and mediated the PAH uptake in the normally treated CGE in rats was particularly noteworthy. These data also correlated with reduced nitric oxide production, highlighting the potential of CGE as a therapeutic agent for managing T2DM-related renal complications.
CONCLUSION
These findings suggest that CGE has antidiabetic effects and directly prevents diabetic nephropathy through oxidative/nitrosative stress pathways.
Keyword
Figure
Reference
-
1. Peerapornpisal Y, Amornledpison D, Rujjanawate C, Ruangrit K, Kanjanapothi D.. Two endemic species of macroalgae in Nan River, Northern Thailand, as therapeutic agents. ScienceAsia. 2006; 32(Suppl 1):71–76.2. Laungsuwon R, Chulalaksananukul W. Antioxidant and anticancer activities of freshwater green algae, Cladophora glomerata and Microspora floccosa, from Nan River in Northern Thailand. Maejo Int J Sci. 2013; 7:181–188.3. Srimaroeng C, Ontawong A, Saowakon N, Vivithanaporn P, Pongchaidecha A, Amornlerdpison D, Soodvilai S, Chatsudthipong V. Antidiabetic and renoprotective effects of Cladophora glomerata Kützing extract in experimental type 2 diabetic rats: a potential nutraceutical product for diabetic nephropathy. J Diabetes Res. 2015; 2015:320167. PMID: 25883984.4. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014; 37(Suppl 1):S81–S90. PMID: 24357215.5. Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001; 414:813–820. PMID: 11742414.6. Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Oxidative stress and stress-activated signaling pathways: a unifying hypothesis of type 2 diabetes. Endocr Rev. 2002; 23:599–622. PMID: 12372842.7. Kanwar YS, Wada J, Sun L, Xie P, Wallner EI, Chen S, Chugh S, Danesh FR. Diabetic nephropathy: mechanisms of renal disease progression. Exp Biol Med (Maywood). 2008; 233:4–11. PMID: 18156300.8. Yu T, Jhun BS, Yoon Y. High-glucose stimulation increases reactive oxygen species production through the calcium and mitogen-activated protein kinase-mediated activation of mitochondrial fission. Antioxid Redox Signal. 2011; 14:425–437. PMID: 20518702.9. Ceriello A, Morocutti A, Mercuri F, Quagliaro L, Moro M, Damante G, Viberti GC. Defective intracellular antioxidant enzyme production in type 1 diabetic patients with nephropathy. Diabetes. 2000; 49:2170–2177. PMID: 11118022.10. Fujii H, Kono K, Nakai K, Goto S, Komaba H, Hamada Y, Shinohara M, Kitazawa R, Kitazawa S, Fukagawa M. Oxidative and nitrosative stress and progression of diabetic nephropathy in type 2 diabetes. Am J Nephrol. 2010; 31:342–352. PMID: 20224273.11. Hiragushi K, Sugimoto H, Shikata K, Yamashita T, Miyatake N, Shikata Y, Wada J, Kumagai I, Fukushima M, Makino H. Nitric oxide system is involved in glomerular hyperfiltration in Japanese normo- and micro-albuminuric patients with type 2 diabetes. Diabetes Res Clin Pract. 2001; 53:149–159. PMID: 11483230.12. Sugimoto H, Shikata K, Matsuda M, Kushiro M, Hayashi Y, Hiragushi K, Wada J, Makino H. Increased expression of endothelial cell nitric oxide synthase (ecNOS) in afferent and glomerular endothelial cells is involved in glomerular hyperfiltration of diabetic nephropathy. Diabetologia. 1998; 41:1426–1434. PMID: 9867209.13. Wada J, Makino H. Inflammation and the pathogenesis of diabetic nephropathy. Clin Sci (Lond). 2013; 124:139–152. PMID: 23075333.14. Ohga S, Shikata K, Yozai K, Okada S, Ogawa D, Usui H, Wada J, Shikata Y, Makino H. Thiazolidinedione ameliorates renal injury in experimental diabetic rats through anti-inflammatory effects mediated by inhibition of NF-κB activation. Am J Physiol Renal Physiol. 2007; 292:F1141–F1150. PMID: 17190910.15. Soetikno V, Sari FR, Veeraveedu PT, Thandavarayan RA, Harima M, Sukumaran V, Lakshmanan AP, Suzuki K, Kawachi H, Watanabe K. Curcumin ameliorates macrophage infiltration by inhibiting NF-κB activation and proinflammatory cytokines in streptozotocin induced-diabetic nephropathy. Nutr Metab (Lond). 2011; 8:35. PMID: 21663638.16. Ha H, Yoon SJ, Kim KH. High glucose can induce lipid peroxidation in the isolated rat glomeruli. Kidney Int. 1994; 46:1620–1626. PMID: 7700020.17. Locke M, Anderson J. NF-κB activation in organs from STZ-treated rats. Appl Physiol Nutr Metab. 2011; 36:121–127. PMID: 21326386.18. Iliescu R, Chade AR. Progressive renal vascular proliferation and injury in obese Zucker rats. Microcirculation. 2010; 17:250–258. PMID: 20536738.19. Hilgendorf C, Ahlin G, Seithel A, Artursson P, Ungell AL, Karlsson J. Expression of thirty-six drug transporter genes in human intestine, liver, kidney, and organotypic cell lines. Drug Metab Dispos. 2007; 35:1333–1340. PMID: 17496207.20. Srimaroeng C, Perry JL, Pritchard JB. Physiology, structure, and regulation of the cloned organic anion transporters. Xenobiotica. 2008; 38:889–935. PMID: 18668434.21. Sharma K, Karl B, Mathew AV, Gangoiti JA, Wassel CL, Saito R, Pu M, Sharma S, You YH, Wang L, et al. Metabolomics reveals signature of mitochondrial dysfunction in diabetic kidney disease. J Am Soc Nephrol. 2013; 24:1901–1912. PMID: 23949796.22. Wang L, Sweet DH. Renal organic anion transporters (SLC22 family): expression, regulation, roles in toxicity, and impact on injury and disease. AAPS J. 2013; 15:53–69. PMID: 23054972.23. Lungkaphin A, Arjinajarn P, Srimaroeng C, Chatsudthipong V. Function and expression of renal organic anion transporters in experimental diabetes in mice. ScienceAsia. 2012; 38:18–23.24. Ontawong A, Saowakon N, Vivithanaporn P, Pongchaidecha A, Lailerd N, Amornlerdpison D, Lungkaphin A, Srimaroeng C. Antioxidant and renoprotective effects of Spirogyra neglecta (Hassall) Kützing extract in experimental type 2 diabetic rats. BioMed Res Int. 2013; 2013:820786. PMID: 23862157.25. Barros SA, Srimaroeng C, Perry JL, Walden R, Dembla-Rajpal N, Sweet DH, Pritchard JB. Activation of protein kinase Cζ increases OAT1 (SLC22A6)- and OAT3 (SLC22A8)-mediated transport. J Biol Chem. 2009; 284:2672–2679. PMID: 19028678.26. Pelletier MM, Kleinbongard P, Ringwood L, Hito R, Hunter CJ, Schechter AN, Gladwin MT, Dejam A. The measurement of blood and plasma nitrite by chemiluminescence: pitfalls and solutions. Free Radic Biol Med. 2006; 41:541–548. PMID: 16863986.27. Yang BK, Vivas EX, Reiter CD, Gladwin MT. Methodologies for the sensitive and specific measurement of S-nitrosothiols, iron-nitrosyls, and nitrite in biological samples. Free Radic Res. 2003; 37:1–10. PMID: 12653211.28. Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008; 22:659–661. PMID: 17942826.29. Busik JV, Mohr S, Grant MB. Hyperglycemia-induced reactive oxygen species toxicity to endothelial cells is dependent on paracrine mediators. Diabetes. 2008; 57:1952–1965. PMID: 18420487.30. Yang RL, Shi YH, Hao G, Li W, Le GW. Increasing oxidative stress with progressive hyperlipidemia in human: relation between malondialdehyde and atherogenic index. J Clin Biochem Nutr. 2008; 43:154–158. PMID: 19015749.31. Boylan JA, Lawrence KA, Downey JS, Gherardini FC. Borrelia burgdorferi membranes are the primary targets of reactive oxygen species. Mol Microbiol. 2008; 68:786–799. PMID: 18373524.32. Guo L, Chen Z, Amarnath V, Davies SS. Identification of novel bioactive aldehyde-modified phosphatidylethanolamines formed by lipid peroxidation. Free Radic Biol Med. 2012; 53:1226–1238. PMID: 22898174.33. Pandey KB, Rizvi SI. Markers of oxidative stress in erythrocytes and plasma during aging in humans. Oxid Med Cell Longev. 2010; 3:2–12. PMID: 20716923.34. Molitch ME, DeFronzo RA, Franz MJ, Keane WF, Mogensen CE, Parving HH. American Diabetes Association. Diabetic nephropathy. Diabetes Care. 2003; 26(Suppl 1):S94–S98. PMID: 12502629.35. Miranda-Díaz AG, Pazarín-Villaseñor L, Yanowsky-Escatell FG, Andrade-Sierra J. Oxidative stress in diabetic nephropathy with early chronic kidney disease. J Diabetes Res. 2016; 2016:7047238. PMID: 27525285.36. Ishii T, Itoh K, Yamamoto M. Roles of Nrf2 in activation of antioxidant enzyme genes via antioxidant responsive elements. Methods Enzymol. 2002; 348:182–190. PMID: 11885271.37. Ighodaro OM, Akinloye OA. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): their fundamental role in the entire antioxidant defence grid. Alexandria J Med. 2018; 54:287–293.38. El-Bahr SM. Curcumin regulates gene expression of insulin like growth factor, B-cell CLL/lymphoma 2 and antioxidant enzymes in streptozotocin induced diabetic rats. BMC Complement Altern Med. 2013; 13:368. PMID: 24364912.39. Iwata K, Nishinaka T, Matsuno K, Yabe-Nishimura C. Increased gene expression of glutathione peroxidase-3 in diabetic mouse heart. Biol Pharm Bull. 2006; 29:1042–1045. PMID: 16651743.40. Limaye PV, Raghuram N, Sivakami S. Oxidative stress and gene expression of antioxidant enzymes in the renal cortex of streptozotocin-induced diabetic rats. Mol Cell Biochem. 2003; 243:147–152. PMID: 12619900.41. Zhou LZ, Johnson AP, Rando TA. NFκB and AP-1 mediate transcriptional responses to oxidative stress in skeletal muscle cells. Free Radic Biol Med. 2001; 31:1405–1416. PMID: 11728812.42. Schreiber J, Jenner RG, Murray HL, Gerber GK, Gifford DK, Young RA. Coordinated binding of NF-κB family members in the response of human cells to lipopolysaccharide. Proc Natl Acad Sci U S A. 2006; 103:5899–5904. PMID: 16595631.43. Arias-Salvatierra D, Silbergeld EK, Acosta-Saavedra LC, Calderon-Aranda ES. Role of nitric oxide produced by iNOS through NF-κB pathway in migration of cerebellar granule neurons induced by Lipopolysaccharide. Cell Signal. 2011; 23:425–435. PMID: 20955790.44. Prabhakar SS. Role of nitric oxide in diabetic nephropathy. Semin Nephrol. 2004; 24:333–344. PMID: 15252773.45. Kanlaya R, Khamchun S, Kapincharanon C, Thongboonkerd V. Protective effect of epigallocatechin-3-gallate (EGCG) via Nrf2 pathway against oxalate-induced epithelial mesenchymal transition (EMT) of renal tubular cells. Sci Rep. 2016; 6:30233. PMID: 27452398.46. Zhang X, He H, Liang D, Jiang Y, Liang W, Chi ZH, Ma J. Protective effects of berberine on renal injury in streptozotocin (STZ)-induced diabetic mice. Int J Mol Sci. 2016; 17:1327. PMID: 27529235.47. Wink DA, Miranda KM, Espey MG, Pluta RM, Hewett SJ, Colton C, Vitek M, Feelisch M, Grisham MB. Mechanisms of the antioxidant effects of nitric oxide. Antioxid Redox Signal. 2001; 3:203–213. PMID: 11396476.48. Harris RC. Growth factors and cytokines in acute renal failure. Adv Ren Replace Ther. 1997; 4:43–53. PMID: 9113240.49. Moriya R, Manivel JC, Mauer M. Juxtaglomerular apparatus T-cell infiltration affects glomerular structure in type 1 diabetic patients. Diabetologia. 2004; 47:82–88. PMID: 14618232.50. Nguyen D, Ping F, Mu W, Hill P, Atkins RC, Chadban SJ. Macrophage accumulation in human progressive diabetic nephropathy. Nephrology (Carlton). 2006; 11:226–231. PMID: 16756636.51. Jung SH, Kim BJ, Lee EH, Osborne NN. Isoquercitrin is the most effective antioxidant in the plant Thuja orientalis and able to counteract oxidative-induced damage to a transformed cell line (RGC-5 cells). Neurochem Int. 2010; 57:713–721. PMID: 20708054.52. Rathore K, Choudhary S, Odoi A, Wang HC. Green tea catechin intervention of reactive oxygen species-mediated ERK pathway activation and chronically induced breast cell carcinogenesis. Carcinogenesis. 2012; 33:174–183. PMID: 22045026.53. VanWert AL, Gionfriddo MR, Sweet DH. Organic anion transporters: discovery, pharmacology, regulation and roles in pathophysiology. Biopharm Drug Dispos. 2010; 31:1–71. PMID: 19953504.54. Song IS, Lee IK, Chung SJ, Kim SG, Lee MG, Shim CK. Effect of nitric oxide on the sinusoidal uptake of organic cations and anions by isolated hepatocytes. Arch Pharm Res. 2002; 25:984–988. PMID: 12510858.55. Maeng HJ, Kim MH, Jin HE, Shin SM, Tsuruo T, Kim SG, Kim DD, Shim CK, Chung SJ. Functional induction of P-glycoprotein in the blood-brain barrier of streptozotocin-induced diabetic rats: evidence for the involvement of nuclear factor-κB, a nitrosative stress-sensitive transcription factor, in the regulation. Drug Metab Dispos. 2007; 35:1996–2005. PMID: 17664251.56. Maeng HJ, Bang YJ, Chung SJ. Functional impairment of P-glycoprotein by sodium nitroprusside pretreatment in mouse brain capillary endothelial cells. Arch Pharm Res. 2012; 35:1215–1221. PMID: 22864744.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Molecular aspects of organic ion transporters in the kidney
- Molecular Physiology of Renal Organic Anion Transporters
- Introduction of Organic Anion Transporters (SLC22A) and a Regulatory Mechanism by Caveolins
- Protective effect of maltol on pathological response of cardiomyocyte in dystrophic mice
- Effects of Increased Uric Acid Intake on the Abundance of Urate-anion exchanger and Organic Anion Transporter Proteins in the Rat Kidney