Anat Cell Biol.
2024 Sep;57(3):384-391. 10.5115/acb.24.050.
Morphological aspects of small intestinal mucosal injury and repair after electron irradiation
- Affiliations
-
- 1Laborant of the Labrotory of Histology and Immunohistochemistry, I.M. Sechenov First Moscow State Medical University (Sechenov University), Moscow, Russia
- 2National Medical Research Centre of Radiology, Ministry of Health of Russia, Moscow, Russia
- 3Federal State Budget Educational Institution of Higher Education A.I. Yevdokimov Moscow State University of Medicine and Dentistry (MSUMD), Moscow, Russia
- 4Research and Educational Resource Center for Immunophenotyping, Digital Spatial Profiling and Ultrastructural Analysis Innovative Technologies, RUDN University, Moscow, Russia
- KMID: 2559754
- DOI: http://doi.org/10.5115/acb.24.050
Abstract
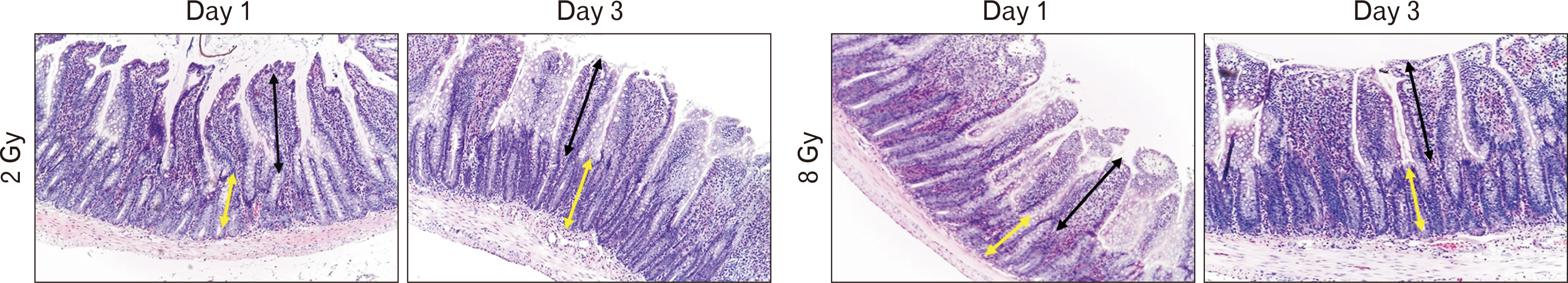
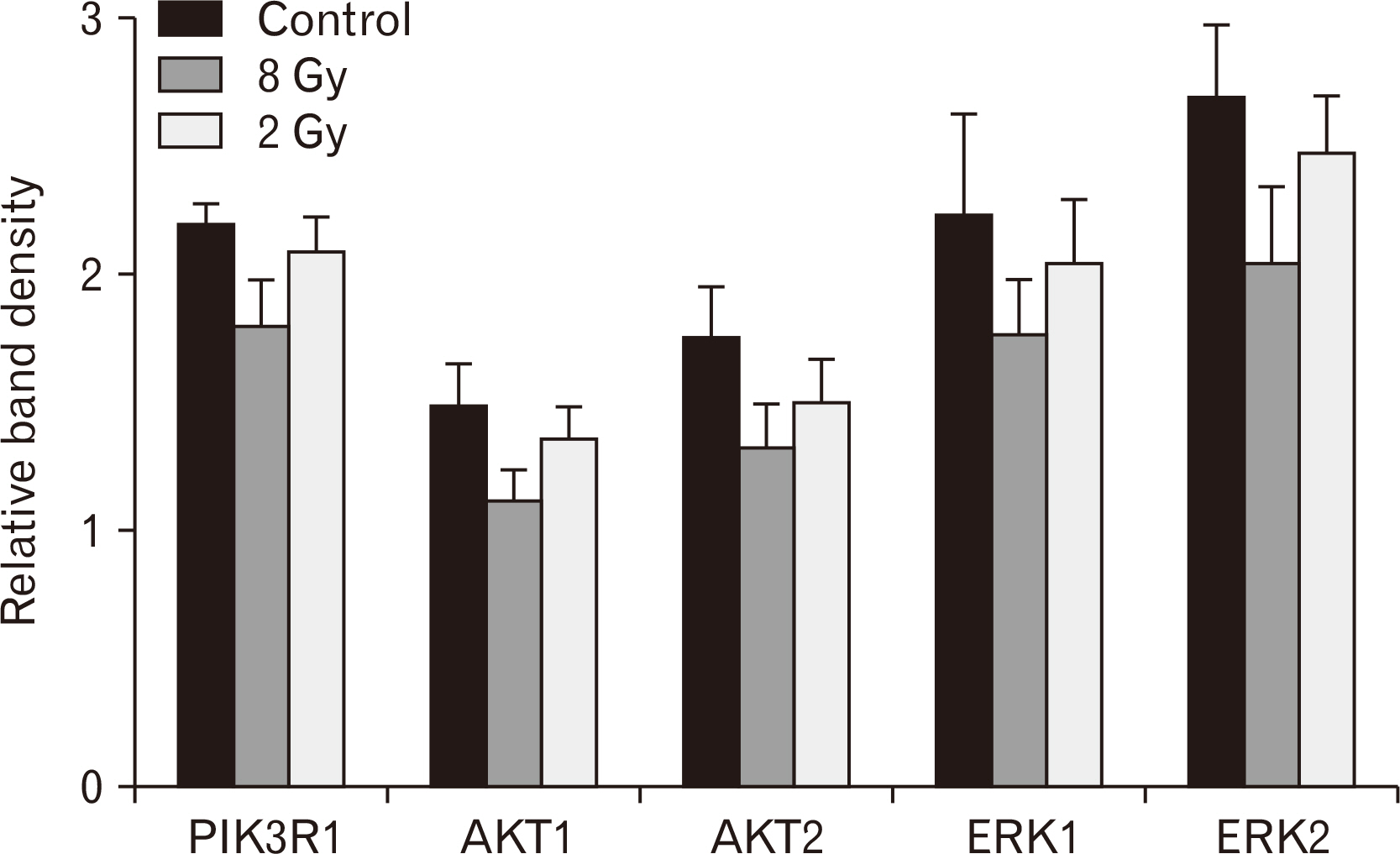
- Morphological evaluation of the small intestine mucosa and apoptosis activity (caspase-3) is necessary to assess the severity of damage to the small intestine. At the same time, proliferative index based on Ki-67 can be used to assess the regenerative potential of the small intestine. Fragments of small intestine of Wistar rats (n=60) of three groups: I) control (n=20); II) experimental group (n=20; local single electron irradiation at a dose of 2 Gy), III) experimental group (n=20; local single electron irradiation at a dose of 8 Gy) were studied by light microscopy using hematoxylin and eosin staining and immunohistochemical reactions with antibodies to Ki-67 and caspase-3. In all samples of the experimental groups, a decrease in all morphometric indices was observed on day 1 with a tendency to recover on day 3. Small intestinal electron irradiation led to disturbances in the histoarchitecture of varying severity, and an increase in cell apoptosis was observed (increased expression of caspase-3 and decrease in Ki-67). In addition, modulation of the PI3K/AKT and MAPK/ERK signaling pathways was detected. The most pronounced destructive changes were observed in the group of 8 Gy single electron irradiation. Local irradiation of the small intestine with electrons at a dose of 2 and 8 Gy results in a decrease in the number of enterocytes, mainly stem cells of the intestinal crypts.
Figure
Reference
-
References
1. Collins JT, Nguyen A, Badireddy M. Anatomy, abdomen and pelvis, small intestine. StatPearls Publishing;2024.2. Park HS, Goodlad RA, Ahnen DJ, Winnett A, Sasieni P, Lee CY, Wright NA. 1997; Effects of epidermal growth factor and dimethylhydrazine on crypt size, cell proliferation, and crypt fission in the rat colon. cell proliferation and crypt fission are controlled independently. Am J Pathol. 151:843–52. PMID: 9284833. PMCID: PMC1857833.3. Livanova AA, Fedorova AA, Zavirsky AV, Bikmurzina AE, Krivoi II, Markov AG. 2021; Dose and time dependence of functional impairments in rat jejunum following ionizing radiation exposure. Physiol Rep. 9:e14960. DOI: 10.14814/phy2.14960. PMID: 34337895. PMCID: PMC8326886.4. Moraes FY, Carvalho Hde A, Hanna SA, Silva JL, Marta GN. 2013; Literature review of clinical results of total skin electron irradiation (TSEBT) of mycosis fungoides in adults. Rep Pract Oncol Radiother. 19:92–8. DOI: 10.1016/j.rpor.2013.08.003. PMID: 24936326. PMCID: PMC4054998.5. Barney BM, Petersen IA, Dowdy SC, Bakkum-Gamez JN, Klein KA, Haddock MG. 2013; Intraoperative electron beam radiotherapy (IOERT) in the management of locally advanced or recurrent cervical cancer. Radiat Oncol. 8:80. DOI: 10.1186/1748-717X-8-80. PMID: 23566444. PMCID: PMC3641982.6. Brzezianska E, Pastuszak-Lewandoska D. 2011; A minireview: the role of MAPK/ERK and PI3K/Akt pathways in thyroid follicular cell-derived neoplasm. Front Biosci (Landmark Ed). 16:422–39. DOI: 10.2741/3696. PMID: 21196179.7. Olsson M, Zhivotovsky B. 2011; Caspases and cancer. Cell Death Differ. 18:1441–9. DOI: 10.1038/cdd.2011.30. PMID: 21455218. PMCID: PMC3178435.8. Beroske L, Van den Wyngaert T, Stroobants S, Van der Veken P, Elvas F. 2021; Molecular Imaging of apoptosis: the case of caspase-3 radiotracers. Int J Mol Sci. 22:3948. DOI: 10.3390/ijms22083948. PMID: 33920463. PMCID: PMC8069194.9. Gerdes J, Schwab U, Lemke H, Stein H. 1983; Production of a mouse monoclonal antibody reactive with a human nuclear antigen associated with cell proliferation. Int J Cancer. 31:13–20. DOI: 10.1002/ijc.2910310104. PMID: 6339421.10. Ishibashi N, Maebayashi T, Aizawa T, Sakaguchi M, Nishimaki H, Masuda S. 2017; Correlation between the Ki-67 proliferation index and response to radiation therapy in small cell lung cancer. Radiat Oncol. 12:16. DOI: 10.1186/s13014-016-0744-1. PMID: 28086989. PMCID: PMC5237196.11. Keam S, MacKinnon KM, D'Alonzo RA, Gill S, Ebert MA, Nowak AK, Cook AM. 2022; Effects of photon radiation on DNA damage, cell proliferation, cell survival, and apoptosis of murine and human mesothelioma cell lines. Adv Radiat Oncol. 7:101013. DOI: 10.1016/j.adro.2022.101013. PMID: 36420194. PMCID: PMC9677206.12. Akedo I, Ishikawa H, Ioka T, Kaji I, Narahara H, Ishiguro S, Suzuki T, Otani T. 2001; Evaluation of epithelial cell proliferation rate in normal-appearing colonic mucosa as a high-risk marker for colorectal cancer. Cancer Epidemiol Biomarkers Prev. 10:925–30. PMID: 11535542.13. Andrés-Sánchez N, Fisher D, Krasinska L. 2022; Physiological functions and roles in cancer of the proliferation marker Ki-67. J Cell Sci. 135:jcs258932. DOI: 10.1242/jcs.258932. PMID: 35674256.14. Blanpain C, Horsley V, Fuchs E. 2007; Epithelial stem cells: turning over new leaves. Cell. 128:445–58. DOI: 10.1016/j.cell.2007.01.014. PMID: 17289566. PMCID: PMC2408375.15. Gordon JI, Hermiston ML. 1994; Differentiation and self-renewal in the mouse gastrointestinal epithelium. Curr Opin Cell Biol. 6:795–803. DOI: 10.1016/0955-0674(94)90047-7. PMID: 7880525.16. Marín A, Martín M, Liñán O, Alvarenga F, López M, Fernández L, Büchser D, Cerezo L. 2014; Bystander effects and radiotherapy. Rep Pract Oncol Radiother. 20:12–21. DOI: 10.1016/j.rpor.2014.08.004. PMID: 25535579. PMCID: PMC4268598.17. Najafi M, Fardid R, Hadadi G, Fardid M. 2014; The mechanisms of radiation-induced bystander effect. J Biomed Phys Eng. 4:163–72. PMID: 25599062. PMCID: PMC4289523.18. Potten CS, Owen G, Roberts SA. 1990; The temporal and spatial changes in cell proliferation within the irradiated crypts of the murine small intestine. Int J Radiat Biol. 57:185–99. DOI: 10.1080/09553009014550431. PMID: 1967288.19. Zhou M, Liu X, Li Z, Huang Q, Li F, Li CY. 2018; Caspase-3 regulates the migration, invasion and metastasis of colon cancer cells. Int J Cancer. 143:921–30. DOI: 10.1002/ijc.31374. PMID: 29524226. PMCID: PMC6204286.20. Potten CS, Grant HK. 1998; The relationship between ionizing radiation-induced apoptosis and stem cells in the small and large intestine. Br J Cancer. 78:993–1003. DOI: 10.1038/bjc.1998.618. PMID: 9792141. PMCID: PMC2063142.21. Miyoshi-Imamura T, Kakinuma S, Kaminishi M, Okamoto M, Takabatake T, Nishimura Y, Imaoka T, Nishimura M, Murakami-Murofushi K, Shimada Y. 2010; Unique characteristics of radiation-induced apoptosis in the postnatally developing small intestine and colon of mice. Radiat Res. 173:310–8. DOI: 10.1667/RR1905.1. PMID: 20199216.22. Seidal T, Edvardsson H. 1999; Expression of c-kit (CD117) and Ki67 provides information about the possible cell of origin and clinical course of gastrointestinal stromal tumours. Histopathology. 34:416–24. DOI: 10.1046/j.1365-2559.1999.00643.x. PMID: 10231416.23. Cheng Y, Dong Y, Hou Q, Wu J, Zhang W, Tian H, Li D. 2019; The protective effects of XH-105 against radiation-induced intestinal injury. J Cell Mol Med. 23:2238–47. DOI: 10.1111/jcmm.14159. PMID: 30663222. PMCID: PMC6378229.24. Li LT, Jiang G, Chen Q, Zheng JN. 2015; Ki67 is a promising molecular target in the diagnosis of cancer (review). Mol Med Rep. 11:1566–72. DOI: 10.3892/mmr.2014.2914. PMID: 25384676.25. Lu L, Li W, Chen L, Su Q, Wang Y, Guo Z, Lu Y, Liu B, Qin S. 2019; Radiation-induced intestinal damage: latest molecular and clinical developments. Future Oncol. 15:4105–18. DOI: 10.2217/fon-2019-0416. PMID: 31746639.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Combined Effect of Adriamycin and Imadiation on the Small Intestinal Villi of Mice
- Histomorphologic Changes of Small Intestinal Mucosa after Irradiation in Rats
- Protective Effects of 5-Androstendiol (5-AED) on Radiation-induced Intestinal Injury
- Totally Extraperitoneal Laparoscopic Repair of Obturator Hernia withPartial Intestinal Obstruction
- Posttraumatic Intestinal Stenosis