Korean J Pain.
2024 Oct;37(4):310-319. 10.3344/kjp.24144.
Low level laser therapy alleviates mechanical allodynia in a postoperative and neuropathic pain model and alters the levels of inflammatory factors in rats
- Affiliations
-
- 1Department of Anesthesiology and Pain Medicine, Chonnam National University Medical School, Gwangju, Korea
- 2Department of Anesthesiology and Pain Medicine, Chonnam National University Hwasun Hospital, Hwasun, Korea
- 3BioMedical Sciences Graduate Program (BMSGP), Chonnam National University Medical School, Hwasun, Korea
- KMID: 2559693
- DOI: http://doi.org/10.3344/kjp.24144
Abstract
- Background
This study aimed to investigate the analgesic and preventive effect of low-level laser therapy (LLLT) on the incisional pain model and spinal nerve ligation (SNL) model in rats and identify the possible mechanisms of action.
Methods
Male Sprague-Dawley rats were used, divided into different treatment groups. The single application group received LLLT before or after skin incision or SNL. The consecutive application group received LLLT for six consecutive days post-incision, three days pre-incision, or three consecutive days pre-SNL. The control group underwent skin incision or SNL without LLLT. The von Frey test was used to quantify the pain associated with mechanical allodynia. Pro-inflammatory cytokine level and alterations in nerve growth factor (NGF) expression were measured by using ELISA and immunohistochemistry, respectively in the skin, muscle of the paw, and spinal cord dorsal horn (SCDH).
Results
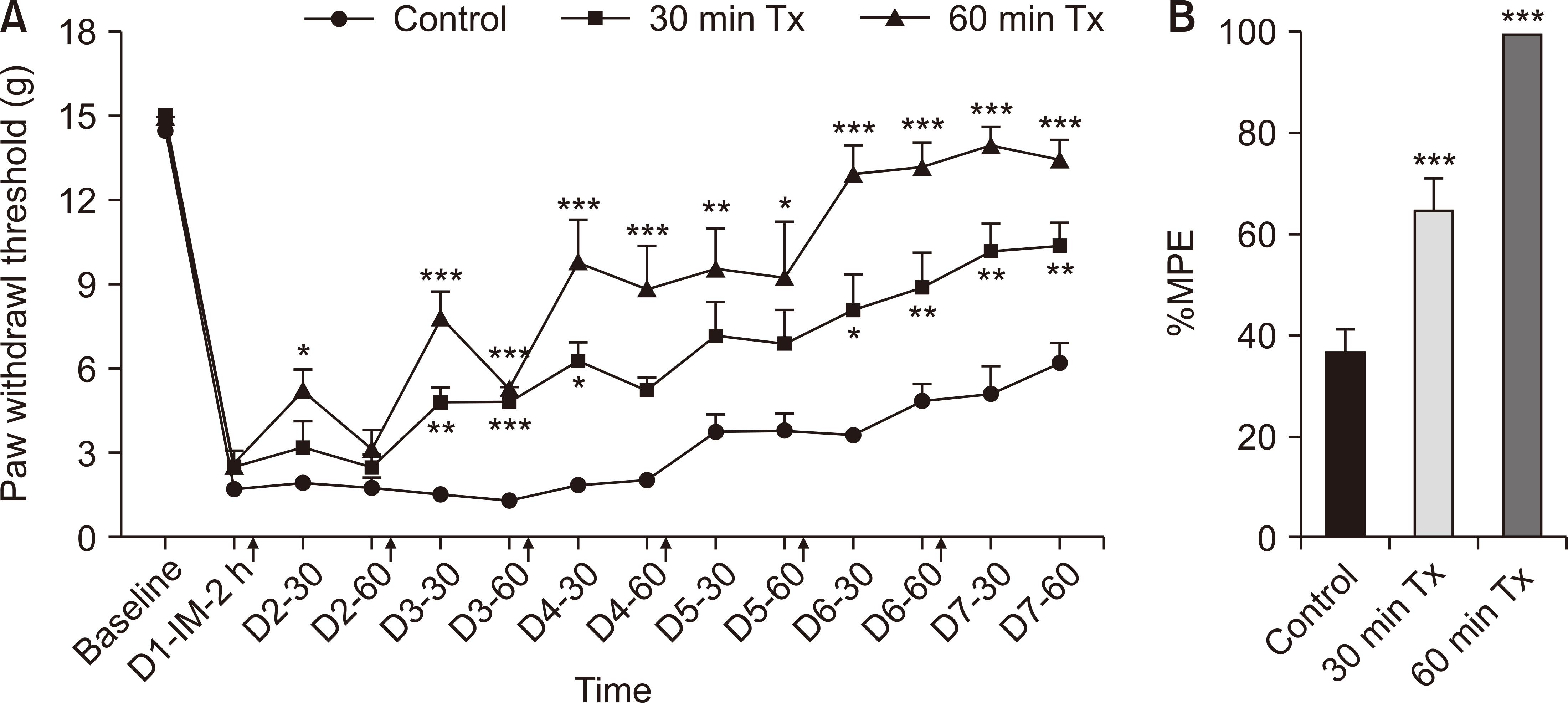
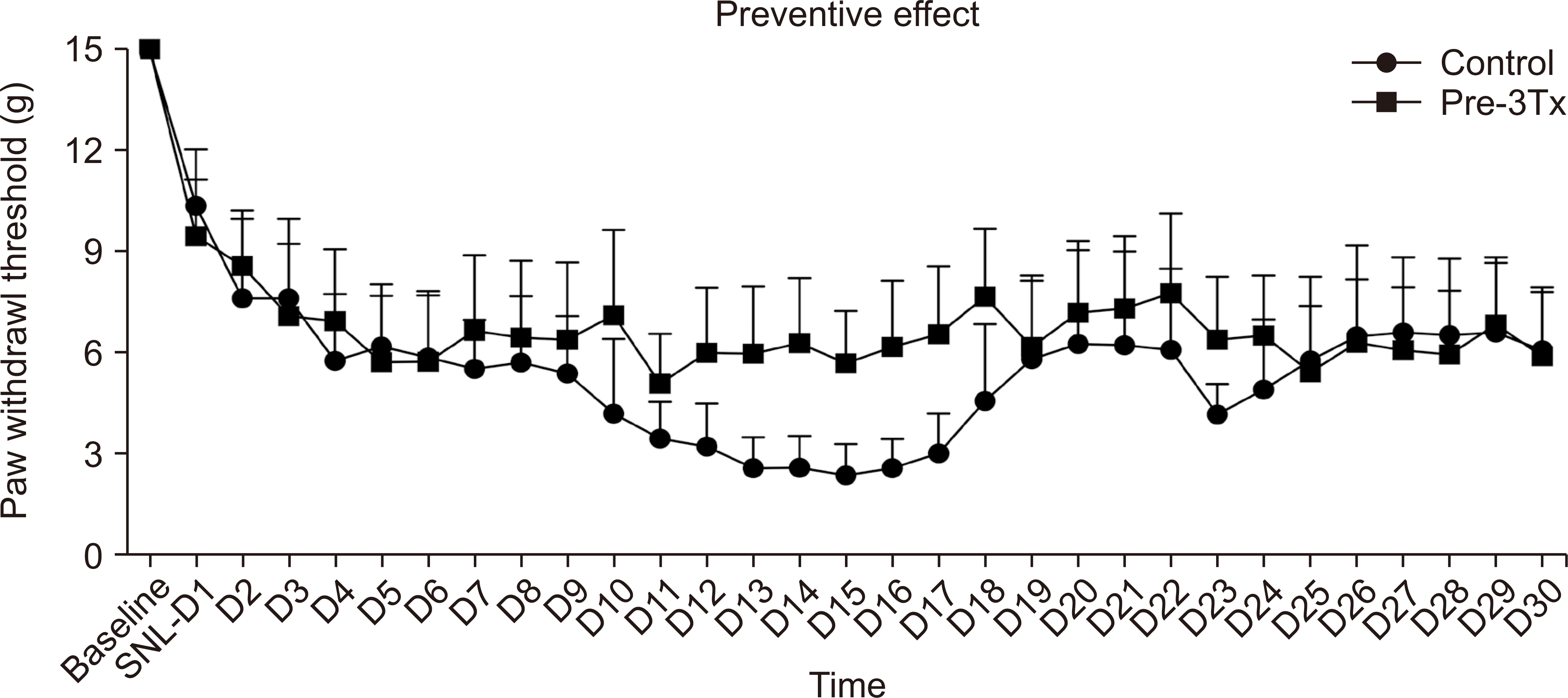
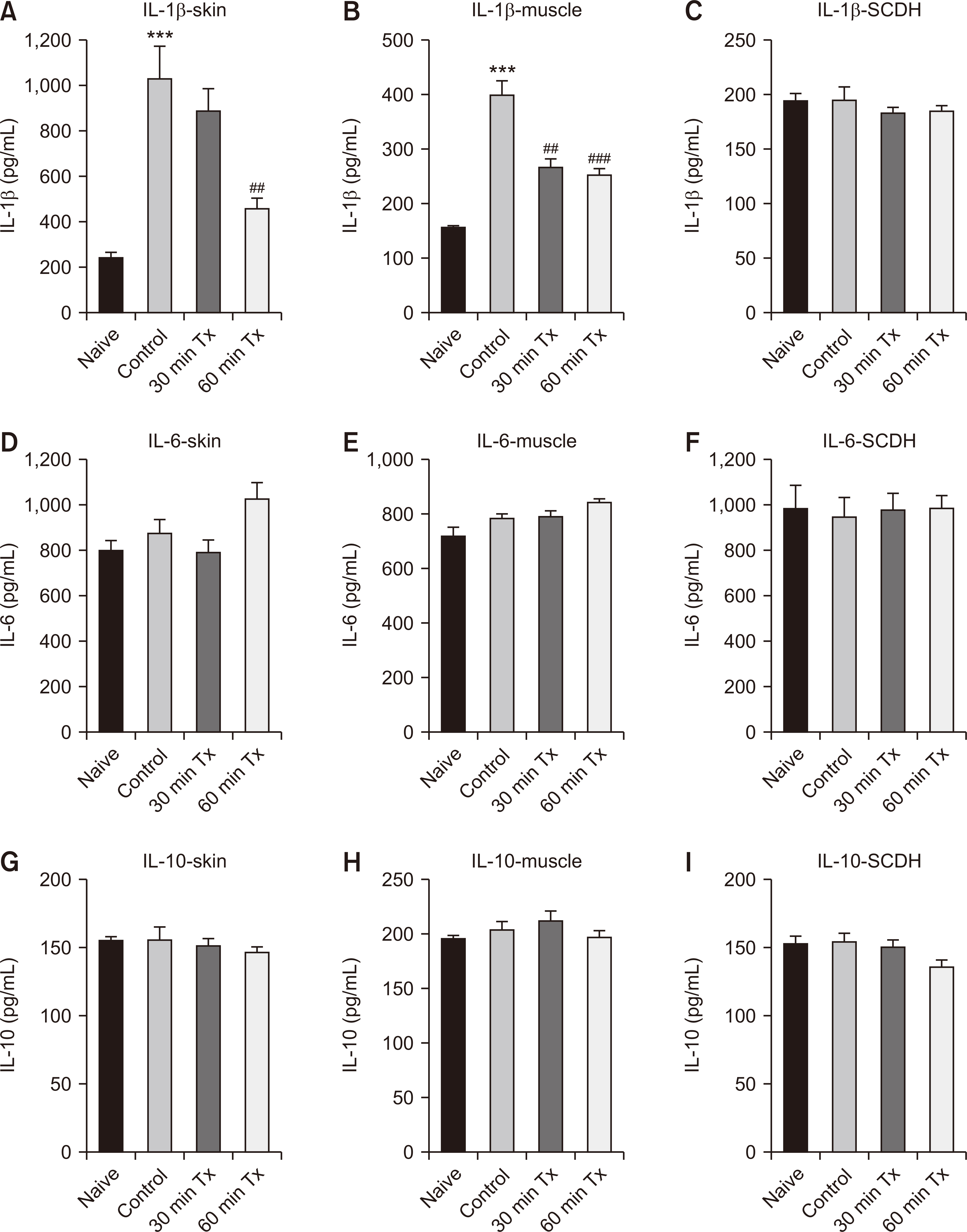
In the incisional pain model, LLLT showed significant analgesic and preventive effect. LLLT ameliorated SNL-induced mechanical allodynia but LLLT had no preventive effect. LLLT decreased interleukin-1β (IL-1β) expression levels in the skin, muscle, and SCDH and reduced the optical density of skin and spinal cord NGF in the incisional pain model.
Conclusions
LLLT alleviated incisional pain and neuropathic pain caused by SNL in rats, and reduced the levels of IL-1β and NGF in the peripheral tissue and SCDH in the incisional pain model. LLLT might be effective in patients with post-operative pain and peripheral neuropathic pain.
Keyword
Figure
Reference
-
1. Pirie K, Traer E, Finniss D, Myles PS, Riedel B. 2022; Current approaches to acute postoperative pain management after major abdominal surgery: a narrative review and future directions. Br J Anaesth. 129:378–93. DOI: 10.1016/j.bja.2022.05.029. PMID: 35803751.2. Cotler HB, Chow RT, Hamblin MR, Carroll J. 2015; The use of low level laser therapy (LLLT) for musculoskeletal pain. MOJ Orthop Rheumatol. 2:00068. DOI: 10.15406/mojor.2015.02.00068. PMID: 26858986. PMCID: PMC4743666.3. Koz G, Kamanli A, Kaban N, Harman H. 2023; Efficacies of extracorporeal shockwave therapy and low-level laser therapy in patients with plantar fasciitis. Foot Ankle Surg. 29:223–7. DOI: 10.1016/j.fas.2023.01.009. PMID: 36737392.4. Falaki F, Nejat AH, Dalirsani Z. 2014; The effect of low-level laser therapy on trigeminal neuralgia: a review of literature. J Dent Res Dent Clin Dent Prospects. 8:1–5. DOI: 10.5681/joddd.2014.001. PMID: 25024832. PMCID: PMC4091693.5. Lazovic M, Ilic-Stojanovic O, Kocic M, Zivkovic V, Hrkovic M, Radosavljevic N. 2014; Placebo-controlled investigation of low-level laser therapy to treat carpal tunnel syndrome. Photomed Laser Surg. 32:336–44. DOI: 10.1089/pho.2013.3563. PMID: 24905929.6. Hung AL, Lim M, Doshi TL. 2017; Targeting cytokines for treatment of neuropathic pain. Scand J Pain. 17:287–93. DOI: 10.1016/j.sjpain.2017.08.002. PMID: 29229214. PMCID: PMC5774983.7. Reis C, Chambel S, Ferreira A, Cruz CD. 2022; Involvement of nerve growth factor (NGF) in chronic neuropathic pain - a systematic review. Rev Neurosci. 34:75–84. DOI: 10.1515/revneuro-2022-0037. PMID: 35792932.8. Dos Anjos LMJ, Salvador PA, de Souza ÁC, de Souza da Fonseca A, de Paoli F, Gameiro J. 2019; Modulation of immune response to induced-arthritis by low-level laser therapy. J Biophotonics. 12:e201800120. DOI: 10.1002/jbio.201800120. PMID: 30203577.9. de Almeida P, Tomazoni SS, Frigo L, de Carvalho Pde T, Vanin AA, Santos LA, et al. 2014; What is the best treatment to decrease pro-inflammatory cytokine release in acute skeletal muscle injury induced by trauma in rats: low-level laser therapy, diclofenac, or cryotherapy? Lasers Med Sci. 29:653–8. DOI: 10.1007/s10103-013-1377-3. PMID: 23812849.10. de Lima FM, Villaverde AB, Albertini R, Corrêa JC, Carvalho RL, Munin E, et al. 2011; Dual Effect of low-level laser therapy (LLLT) on the acute lung inflammation induced by intestinal ischemia and reperfusion: action on anti- and pro-inflammatory cytokines. Lasers Surg Med. 43:410–20. DOI: 10.1002/lsm.21053. PMID: 21674546.11. Brennan TJ, Vandermeulen EP, Gebhart GF. 1996; Characterization of a rat model of incisional pain. Pain. 64:493–502. DOI: 10.1016/0304-3959(95)01441-1. PMID: 8783314.12. Deuis JR, Dvorakova LS, Vetter I. 2017; Methods used to evaluate pain behaviors in rodents. Front Mol Neurosci. 10:284. DOI: 10.3389/fnmol.2017.00284. PMID: 28932184. PMCID: PMC5592204.13. Ho Kim S, Mo Chung J. 1992; An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 50:355–63. DOI: 10.1016/0304-3959(92)90041-9. PMID: 1333581.14. Bjordal JM, Lopes-Martins RA, Joensen J, Couppe C, Ljunggren AE, Stergioulas A, et al. 2008; A systematic review with procedural assessments and meta-analysis of low level laser therapy in lateral elbow tendinopathy (tennis elbow). BMC Musculoskelet Disord. 9:75. DOI: 10.1186/1471-2474-9-75. PMID: 18510742. PMCID: PMC2442599.15. Neto FCJ, Martimbianco ALC, de Andrade RP, Bussadori SK, Mesquita-Ferrari RA, Fernandes KPS. 2020; Effects of photobiomodulation in the treatment of fractures: a systematic review and meta-analysis of randomized clinical trials. Lasers Med Sci. 35:513–22. DOI: 10.1007/s10103-019-02779-4. PMID: 30982176.16. Dos Santos SA, Sampaio LM, Caires JR, Fernandes GHC, Marsico A, Serra AJ, et al. 2019; Parameters and effects of photobiomodulation in plantar fasciitis: a meta-analysis and systematic review. Photobiomodul Photomed Laser Surg. 37:327–35. DOI: 10.1089/photob.2018.4588. PMID: 31107161.17. Okita S, Sasaki R, Kondo Y, Sakamoto J, Honda Y, Okita M. 2023; Effects of low-level laser therapy on inflammatory symptoms in an arthritis rat model. J Phys Ther Sci. 35:55–9. DOI: 10.1589/jpts.35.55. PMID: 36628144. PMCID: PMC9822828.18. Dias FJ, Fazan VPS, Cury DP, de Almeida SRY, Borie E, Fuentes R, et al. 2019; Growth factors expression and ultrastructural morphology after application of low-level laser and natural latex protein on a sciatic nerve crush-type injury. PLoS One. 14:e0210211. DOI: 10.1371/journal.pone.0210211. PMID: 30625210. PMCID: PMC6326513.19. Hsieh YL, Chou LW, Chang PL, Yang CC, Kao MJ, Hong CZ. 2012; Low-level laser therapy alleviates neuropathic pain and promotes function recovery in rats with chronic constriction injury: possible involvements in hypoxia-inducible factor 1α (HIF-1α). J Comp Neurol. 520:2903–16. DOI: 10.1002/cne.23072. PMID: 22351621.20. Lee HG, Park SK, Yoon MH. 2010; Potentiation of morphine antiallodynic efficacy by ACPT-III, a group III metabotropic glutamate receptor agonist, in rat spinal nerve ligation-induced neuropathic pain. Pharmacol Biochem Behav. 96:108–13. DOI: 10.1016/j.pbb.2010.04.014. PMID: 20434481.21. Carvalho B, Clark DJ, Angst MS. 2008; Local and systemic release of cytokines, nerve growth factor, prostaglandin E2, and substance P in incisional wounds and serum following cesarean delivery. J Pain. 9:650–7. DOI: 10.1016/j.jpain.2008.02.004. PMID: 18394968.22. Binshtok AM, Wang H, Zimmermann K, Amaya F, Vardeh D, Shi L, et al. 2008; Nociceptors are interleukin-1beta sensors. J Neurosci. 28:14062–73. DOI: 10.1523/JNEUROSCI.3795-08.2008. PMID: 19109489. PMCID: PMC2690713.23. Kellesarian SV, Malignaggi VR, Majoka HA, Al-Kheraif AA, Kellesarian TV, Romanos GE, et al. 2017; Effect of laser-assisted scaling and root planing on the expression of pro-inflammatory cytokines in the gingival crevicular fluid of patients with chronic periodontitis: a systematic review. Photodiagnosis Photodyn Ther. 18:63–77. DOI: 10.1016/j.pdpdt.2017.02.010. PMID: 28232271.24. Nambi G. 2021; Does low level laser therapy has effects on inflammatory biomarkers IL-1β, IL-6, TNF-α, and MMP-13 in osteoarthritis of rat models-a systemic review and meta-analysis. Lasers Med Sci. 36:475–84. DOI: 10.1007/s10103-020-03124-w. PMID: 32833088.25. Barker PA, Mantyh P, Arendt-Nielsen L, Viktrup L, Tive L. 2020; Nerve growth factor signaling and its contribution to pain. J Pain Res. 13:1223–41. DOI: 10.2147/JPR.S247472. PMID: 32547184. PMCID: PMC7266393.26. Rukwied R, Mayer A, Kluschina O, Obreja O, Schley M, Schmelz M. 2010; NGF induces non-inflammatory localized and lasting mechanical and thermal hypersensitivity in human skin. Pain. 148:407–13. DOI: 10.1016/j.pain.2009.11.022. PMID: 20022698.27. Nicol GD, Vasko MR. 2007; Unraveling the story of NGF-mediated sensitization of nociceptive sensory neurons: ON or OFF the Trks? Mol Interv. 7:26–41. DOI: 10.1124/mi.7.1.6. PMID: 17339604.28. Hoheisel U, Unger T, Mense S. 2005; Excitatory and modulatory effects of inflammatory cytokines and neurotrophins on mechanosensitive group IV muscle afferents in the rat. Pain. 114:168–76. DOI: 10.1016/j.pain.2004.12.020. PMID: 15733642.29. Hayashi K, Shiozawa S, Ozaki N, Mizumura K, Graven-Nielsen T. 2013; Repeated intramuscular injections of nerve growth factor induced progressive muscle hyperalgesia, facilitated temporal summation, and expanded pain areas. Pain. 154:2344–52. DOI: 10.1016/j.pain.2013.07.007. PMID: 23867729.30. Yazdani SO, Golestaneh AF, Shafiee A, Hafizi M, Omrani HA, Soleimani M. 2012; Effects of low level laser therapy on proliferation and neurotrophic factor gene expression of human schwann cells in vitro. J Photochem Photobiol B. 107:9–13. DOI: 10.1016/j.jphotobiol.2011.11.001. PMID: 22178388.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- BDNF/TrkB Signaling Inhibition Suppresses Astrogliosis and Alleviates Mechanical Allodynia in a Partial Crush Injury Model
- Effects of Cervical Sympathectomy on Mechanical Allodynia and Cold Allodynia in a Rat Model of Neuropathic Pain
- Effect of Chemical Sympathectomy and Laser Radiation in the Neuropathic Pain
- Pharmacological interactions between intrathecal pregabalin plus tianeptine or clopidogrel in a rat model of neuropathic pain
- The Effect of Nitric Oxide on Mechanical and Theraml Allodynia in Neuropathic Pain Model of Rat