J Liver Cancer.
2024 Sep;24(2):224-233. 10.17998/jlc.2024.05.12.
Downstaging with atezolizumab-bevacizumab: a case series
- Affiliations
-
- 1Department of Hepatology, AIG Hospitals, Hyderabad, India
- 2Department of Liver Transplant Surgery, AIG Hospitals, Hyderabad, India
- 3Department of Pathology, AIG Hospitals, Hyderabad, India
- 4Department of Liver Transplant Anaesthesia, AIG Hospitals, Hyderabad, India
- 5Department of Medicine, UT Southwestern Medical Centre, Dallas, TX, USA
- KMID: 2559469
- DOI: http://doi.org/10.17998/jlc.2024.05.12
Abstract
- Backgrounds/Aims
Hepatocellular carcinoma (HCC) is generally diagnosed at an advanced stage, which limits curative treatment options for these patients. Locoregional therapy (LRT) is the standard approach to bridge and downstage unresectable HCC for liver transplantation (LT). Atezolizumab-bevacizumab (atezo-bev) can induce objective responses in nearly one-third of patients; however, the role and outcomes of downstaging using atezo-bev remains unknown.
Methods
In this retrospective single-center study, we included consecutive patients between November 2020 and August 2023, who received atezo-bev with or without LRT and were subsequently considered for resection/LT after downstaging.
Results
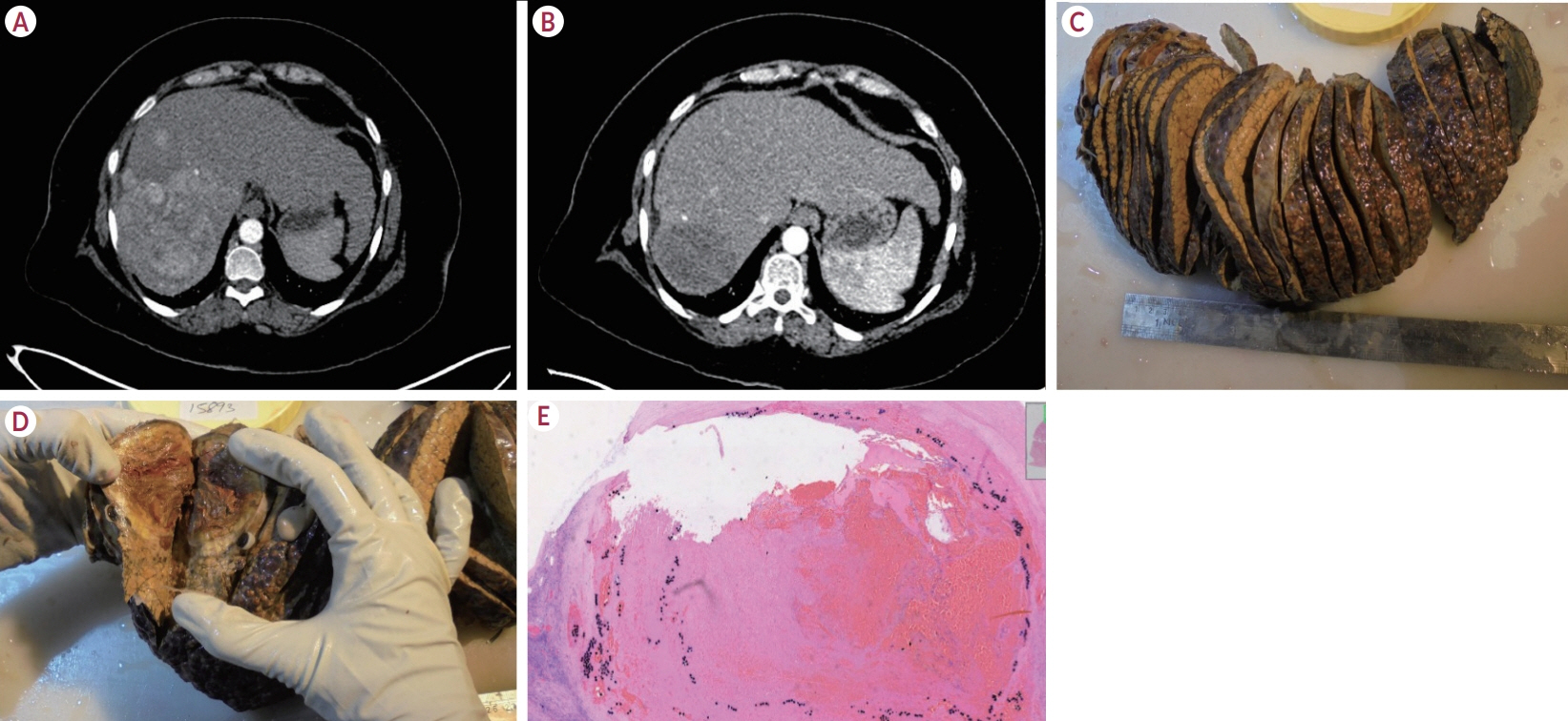
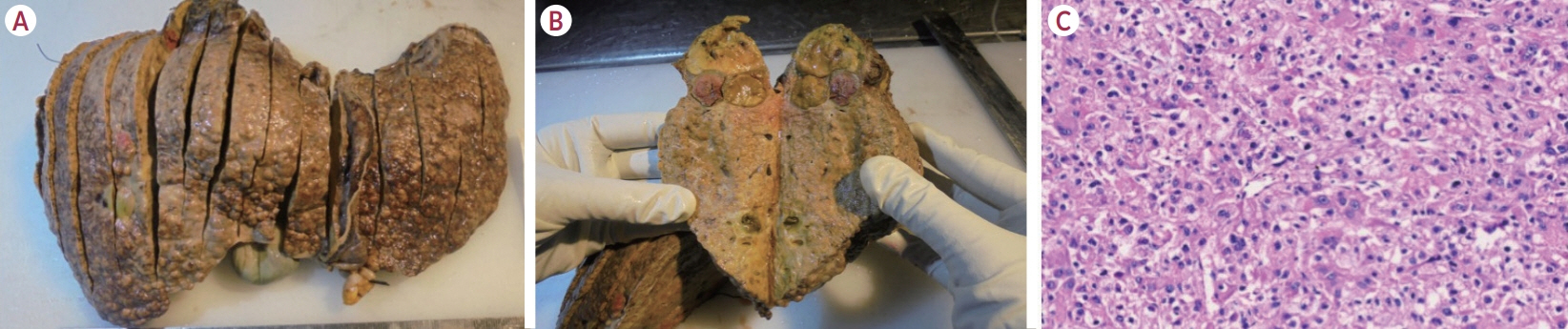
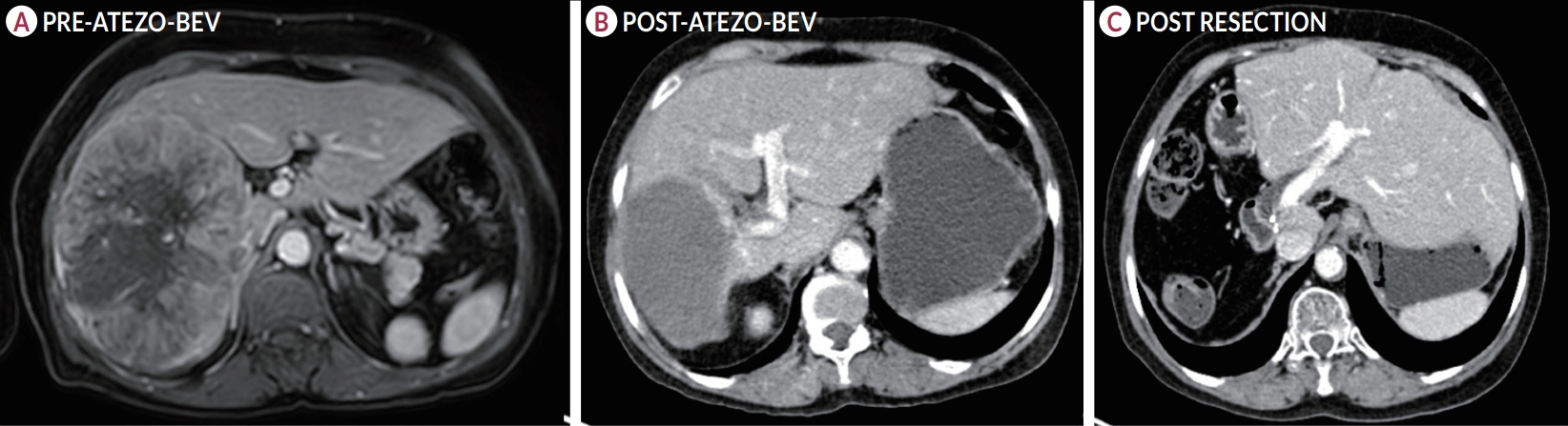
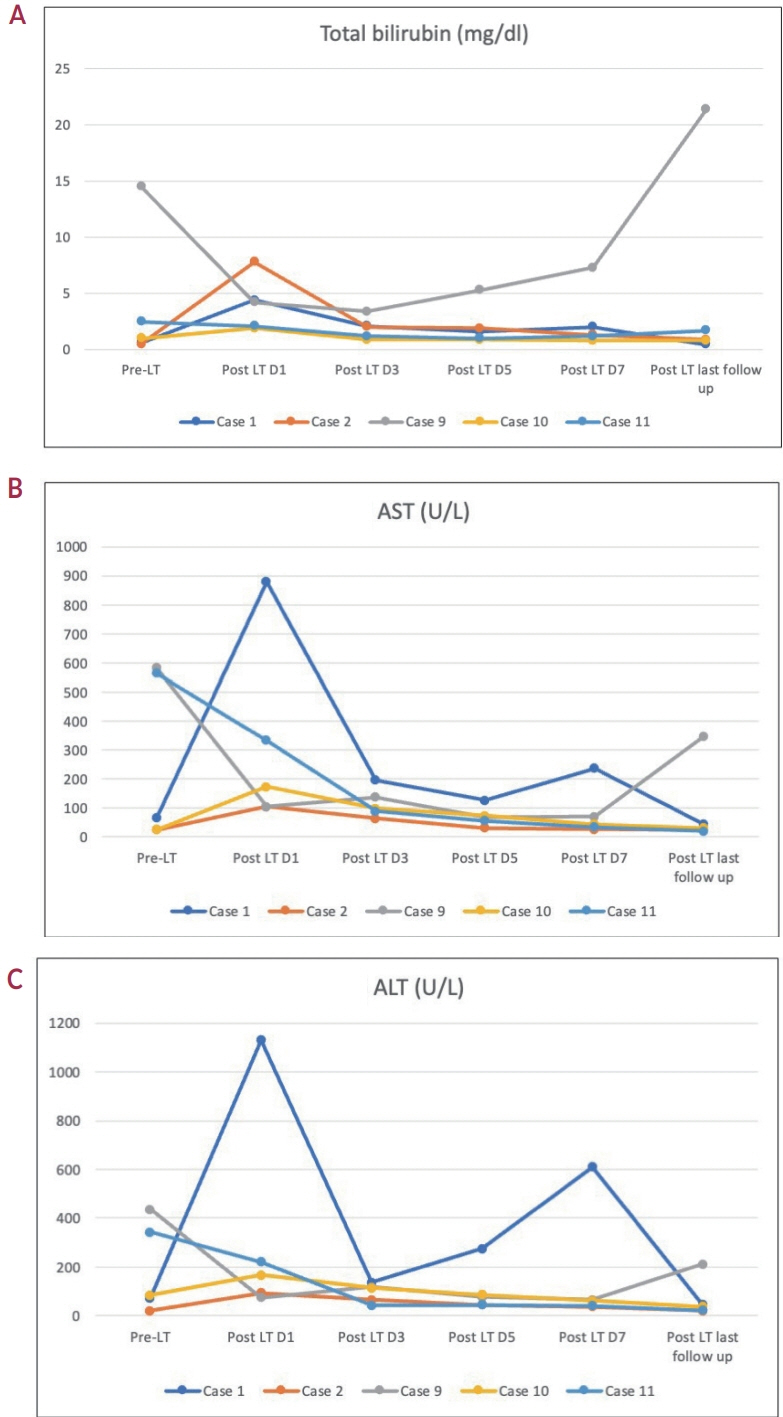
Of the 115 patients who received atezo-bev, 12 patients (10.4%) achieved complete or partial response and were willing to undergo LT; they (age, 58.5 years; women, 17%; Barcelona Clinic Liver Cancer stage system B/C, 5/7) had received 3-12 cycles of atezo- bev, and four of them had received prior LRT. Three patients died before LT, while three were awaiting LT. Six patients underwent curative therapies: four underwent living donor LT after a median of 79.5 days (range, 54-114) following the last atezo-bev dose, one underwent deceased donor LT 38 days after the last dose, and one underwent resection. All but one patient had complete pathologic response with no viable HCC. Three patients experienced wound healing complications, and one required re-exploration and succumbed to sepsis. After a median follow-up of 10 months (range, 4-30), none of the alive patients developed HCC recurrence or graft rejection.
Conclusions
Surgical therapy, including LT, is possible after atezo-bev therapy in well-selected patients after downstaging.
Figure
Reference
-
References
1. Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, Garcia-Criado Á, et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. 2022; 76:681–693.
Article2. Singal AG, Llovet JM, Yarchoan M, Mehta N, Heimbach JK, Dawson LA, et al. AASLD practice guidance on prevention, diagnosis, and treatment of hepatocellular carcinoma. Hepatology. 2023; 78:1922–1965.
Article3. Tabrizian P, Florman SS, Schwartz ME. PD-1 inhibitor as bridge therapy to liver transplantation? Am J Transplant. 2021; 21:1979–1980.4. Schnickel GT, Fabbri K, Hosseini M, Misel M, Berumen J, Parekh J, et al. Liver transplantation for hepatocellular carcinoma following checkpoint inhibitor therapy with nivolumab. Am J Transplant. 2022; 22:1699–1704.
Article5. Au KP, Chok KSH. Immunotherapy after liver transplantation: where are we now? World J Gastrointest Surg. 2021; 13:1267–1278.
Article6. Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020; 382:1894–1905.
Article7. Kulkarni AV, Tevethia H, Kumar K, Premkumar M, Muttaiah MD, Hiraoka A, et al. Effectiveness and safety of atezolizumab-bevacizumab in patients with unresectable hepatocellular carcinoma: a systematic review and meta-analysis. EClinicalMedicine. 2023; 63:102179.
Article8. Abdelrahim M, Esmail A, Umoru G, Westhart K, Abudayyeh A, Saharia A, et al. Immunotherapy as a neoadjuvant therapy for a patient with hepatocellular carcinoma in the pretransplant setting: a case report. Curr Oncol. 2022; 29:4267–4273.9. Chouik Y, Erard D, Demian H, Schulz T, Mazard T, Hartig-Lavie K, et al. Case report: successful liver transplantation after achieving complete clinical remission of advanced HCC with atezolizumab plus bevacizumab combination therapy. Front Immunol. 2023; 14:1205997.
Article10. Schmiderer A, Zoller H, Niederreiter M, Effenberger M, Oberhuber G, Krendl FJ, et al. Liver transplantation after successful downstaging of a locally advanced hepatocellular carcinoma with systemic therapy. Dig Dis. 2023; 41:641–644.
Article11. Kulkarni AV, Krishna V, Kumar K, Sharma M, Patodiya B, Khan A, et al. Safety and efficacy of atezolizumab-bevacizumab in real world: the first indian experience. J Clin Exp Hepatol. 2023; 13:618–623.
Article12. Abou-Alfa GK, Lau G, Kudo M, Chan SL, Kelley RK, Furuse J, et al. Tremelimumab plus durvalumab in unresectable hepatocellular carcinoma. NEJM Evid. 2022; 1:EVIDoa2100070.
Article13. Qin S, Chen M, Cheng AL, Kaseb AO, Kudo M, Lee HC, et al. Atezolizumab plus bevacizumab versus active surveillance in patients with resected or ablated high-risk hepatocellular carcinoma (IMbrave050): a randomised, open-label, multicentre, phase 3 trial. Lancet. 2023; 402:1835–1847.14. Sangro B, Kudo M, Qin S, Ren Z, Chan S, Joseph E, et al. P-347 A phase 3, randomized, double-blind, placebo-controlled study of transarterial chemoembolization combined with durvalumab or durvalumab plus bevacizumab therapy in patients with locoregional hepatocellular carcinoma: EMERALD-1. Ann Oncol. 2020; 31 Suppl 3:S202–S203.15. Zhu AX, Abbas AR, de Galarreta MR, Guan Y, Lu S, Koeppen H, et al. Molecular correlates of clinical response and resistance to atezolizumab in combination with bevacizumab in advanced hepatocellular carcinoma. Nat Med. 2022; 28:1599–1611.16. Marron TU, Fiel MI, Hamon P, Fiaschi N, Kim E, Ward SC, et al. Neoadjuvant cemiplimab for resectable hepatocellular carcinoma: a single-arm, open-label, phase 2 trial. Lancet Gastroenterol Hepatol. 2022; 7:219–229.17. Amado V, González-Rubio S, Zamora J, Alejandre R, Espejo-Cruz ML, Linares C, et al. Clearance of circulating tumor cells in patients with hepatocellular carcinoma undergoing surgical resection or liver transplantation. Cancers (Basel). 2021; 13:2476.18. Court CM, Hou S, Winograd P, Segel NH, Li QW, Zhu Y, et al. A novel multimarker assay for the phenotypic profiling of circulating tumor cells in hepatocellular carcinoma. Liver Transpl. 2018; 24:946–960.19. Limousin W, Laurent-Puig P, Ziol M, Ganne-Carrié N, Nahon P, Ait-Omar A, et al. Molecular-based targeted therapies in patients with hepatocellular carcinoma and hepato-cholangiocarcinoma refractory to atezolizumab/bevacizumab. J Hepatol. 2023; 79:1450–1458.20. Jahagirdar V, Rama K, Habeeb MF, Sharma M, Rao PN, Reddy DN. Systemic therapies for hepatocellular carcinoma in India. J Clin Exp Hepatol;Forthcoming 2024.21. Bao P, Kodra A, Tomic-Canic M, Golinko MS, Ehrlich HP, Brem H. The role of vascular endothelial growth factor in wound healing. J Surg Res. 2009; 153:347–358.
Article22. Johnson KE, Wilgus TA. Vascular endothelial growth factor and angiogenesis in the regulation of cutaneous wound repair. Adv Wound Care (New Rochelle). 2014; 3:647–661.
Article23. Khunger M, Rakshit S, Pasupuleti V, Hernandez AV, Mazzone P, Stevenson J, et al. Incidence of pneumonitis with use of programmed death 1 and programmed death-ligand 1 inhibitors in non-small cell lung cancer: a systematic review and meta-analysis of trials. Chest. 2017; 152:271–281.24. Kulkarni AV, Premkumar M, Arab JP, Kumar K, Sharma M, Reddy ND, et al. Early diagnosis and prevention of infections in cirrhosis. Semin Liver Dis. 2022; 42:293–312.25. Vogel A, Cervantes A, Chau I, Daniele B, Llovet JM, Meyer T, et al. Hepatocellular carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018; 29 Suppl 4:iv238–iv255.26. European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018; 69:182–236.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Atezolizumab and bevacizumab for hepatocellular carcinoma: How to approach salvage therapy for non-responders?: Editorial on “Sorafenib vs. Lenvatinib in advanced hepatocellular carcinoma after atezolizumab/bevacizumab failure: A real-world study”
- A Case of Lichenoid Drug Eruption Associated with Atezolizumab Plus Bevacizumab
- Concurrent transarterial radioembolization and combination atezolizumab/ bevacizumab treatment of infiltrative hepatocellular carcinoma with portal vein tumor thrombosis: a case report
- Clinical significance of the discrepancy between radiological findings and biochemical responses in atezolizumab plus bevacizumab for hepatocellular carcinoma
- A Case of Transverse Myelitis Following Treatment with Atezolizumab for Advanced Hepatocellular Carcinoma