Korean J Gastroenterol.
2023 Jul;82(1):35-39. 10.4166/kjg.2023.054.
A Case of Transverse Myelitis Following Treatment with Atezolizumab for Advanced Hepatocellular Carcinoma
- Affiliations
-
- 1Departments of Internal Medicine, Dong-A University College of Medicine, Busan, Korea
- 2Departments of Neurology, Dong-A University College of Medicine, Busan, Korea
- KMID: 2544938
- DOI: http://doi.org/10.4166/kjg.2023.054
Abstract
- The results of the IMbrave150 study have led to widespread use of the combination therapy of atezolizumab and bevacizumab as a first-line treatment for unresectable or metastatic hepatocellular carcinoma (HCC). Compared to traditional cytotoxic chemotherapy agents, immune checkpoint inhibitors show a spectrum of side effects ranging from mild side effects such as skin rash to potentially severe systemic effects such as myocarditis. We present a case of transverse myelitis diagnosed during the treatment of HCC with atezolizumab and bevacizumab combination therapy.
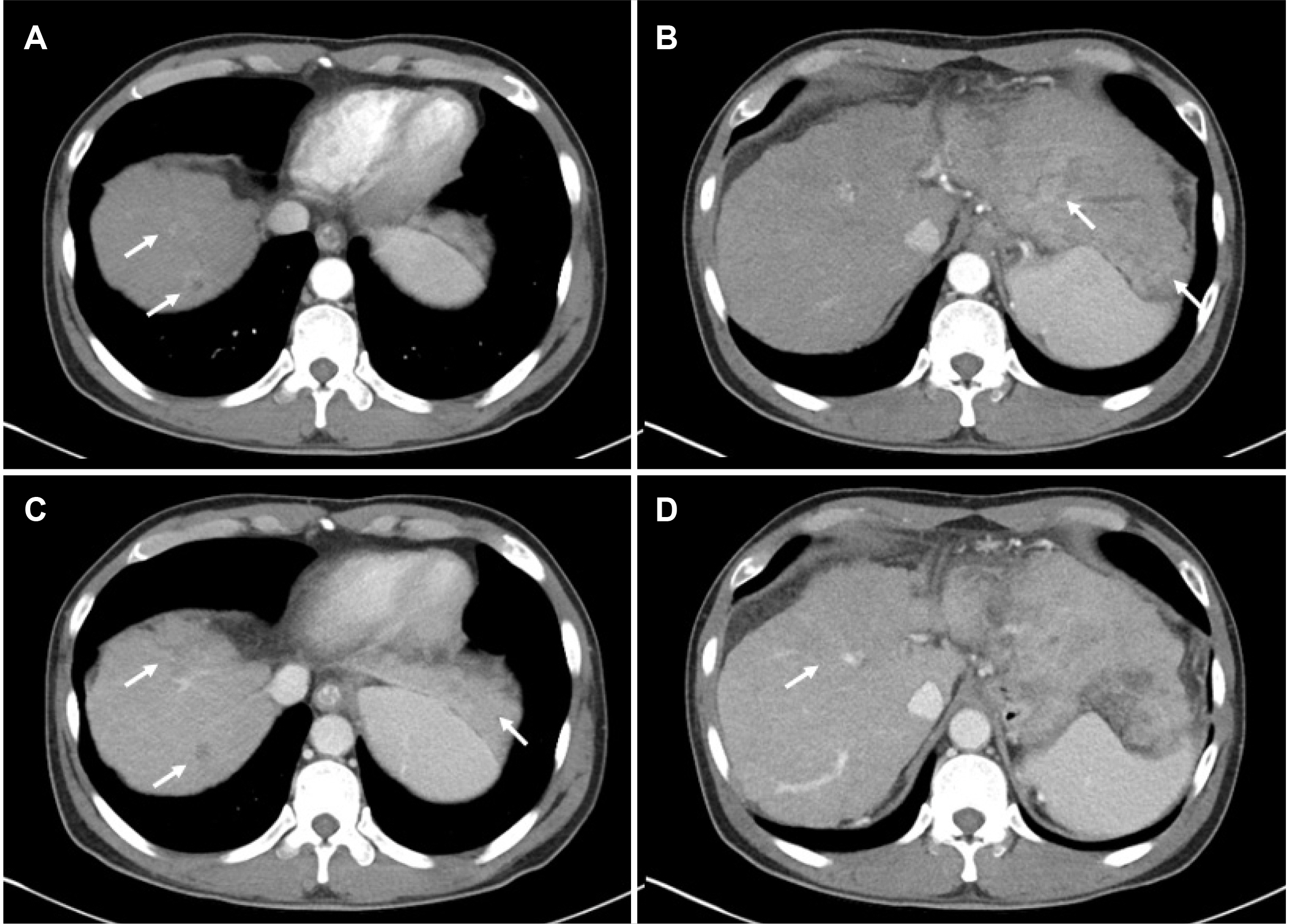
Figure
Reference
-
1. Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR. 2019; A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. 16:589–604. DOI: 10.1038/s41575-019-0186-y. PMID: 31439937. PMCID: PMC6813818.2. Llovet JM, Kelley RK, Villanueva A, et al. 2021; Hepatocellular carcinoma. Nat Rev Dis Primers. 7:6. DOI: 10.1038/s41572-020-00240-3. PMID: 33479224.3. Finn RS, Qin S, Ikeda M, et al. 2020; Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 382:1894–1905. DOI: 10.1056/NEJMoa1915745. PMID: 32402160.4. Korean Liver Cancer Association (KLCA) and National Cancer Center (NCC) Korea. 2022; 2022 KLCA-NCC Korea practice guidelines for the management of hepatocellular carcinoma. Clin Mol Hepatol. 28:583–705. DOI: 10.3350/cmh.2022.0294. PMID: 36263666. PMCID: PMC9597235.5. Su GL, Altayar O, O'Shea R, et al. 2022; AGA clinical practice guideline on systemic therapy for hepatocellular carcinoma. Gastroenterology. 162:920–934. DOI: 10.1053/j.gastro.2021.12.276. PMID: 35210014.6. Reig M, Forner A, Rimola J, et al. 2022; BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol. 76:681–693. DOI: 10.1016/j.jhep.2021.11.018. PMID: 34801630. PMCID: PMC8866082.7. Martins F, Sofiya L, Sykiotis GP, et al. 2019; Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat Rev Clin Oncol. 16:563–580. DOI: 10.1038/s41571-019-0218-0. PMID: 31092901.8. Li C, Bhatti SA, Ying J. 2022; Immune checkpoint inhibitors-associated cardiotoxicity. Cancers (Basel). 14:1145. DOI: 10.3390/cancers14051145. PMID: 35267453. PMCID: PMC8909315.9. Cuzzubbo S, Javeri F, Tissier M, et al. 2017; Neurological adverse events associated with immune checkpoint inhibitors: Review of the literature. Eur J Cancer. 73:1–8. DOI: 10.1016/j.ejca.2016.12.001. PMID: 28064139.10. Yoon JS, Lee HA, Kim HY, et al. 2021; Hepatocellular carcinoma in Korea: An analysis of the 2015 Korean nationwide cancer registry. J Liver Cancer. 21:58–68. Retracted and republished in : J Liver Cancer 2022;22:207. DOI: 10.17998/jlc.21.1.58. PMID: 37384267. PMCID: PMC10035724.11. Cheng AL, Kang YK, Chen Z, et al. 2009; Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 10:25–34. DOI: 10.1016/S1470-2045(08)70285-7. PMID: 19095497.12. Postow MA, Sidlow R, Hellmann MD. 2018; Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. 378:158–168. DOI: 10.1056/NEJMra1703481. PMID: 29320654.13. Beh SC, Greenberg BM, Frohman T, Frohman EM. 2013; Transverse myelitis. Neurol Clin. 31:79–138. DOI: 10.1016/j.ncl.2012.09.008. PMID: 23186897. PMCID: PMC7132741.14. Kitley JL, Leite MI, George JS, Palace JA. 2012; The differential diagnosis of longitudinally extensive transverse myelitis. Mult Scler. 18:271–285. DOI: 10.1177/1352458511406165. PMID: 21669935.15. Kim NH. 2016; Differential diagnosis between multiple sclerosis and neuromyelitis optica spectrum disorder. J Korean Neurol Assoc. 34:290–296. DOI: 10.17340/jkna.2016.4.2.16. Moodie T, Alshaqi O, Alchaki A. 2022; Longitudinal extensive transverse myelitis after chemoradiation therapy with durvalumab, a rare complication: case report. BMC Neurol. 22:107. DOI: 10.1186/s12883-022-02576-7. PMID: 35305566. PMCID: PMC8933995.17. Narumi Y, Yoshida R, Minami Y, et al. 2018; Neuromyelitis optica spectrum disorder secondary to treatment with anti-PD-1 antibody nivolumab: the first report. BMC Cancer. 18:95. DOI: 10.1186/s12885-018-3997-2. PMID: 29361915. PMCID: PMC5781276.18. Esechie A, Fang X, Banerjee P, Rai P, Thottempudi N. A case report of longitudinal extensive transverse myelitis: immunotherapy related adverse effect vs. COVID-19 related immunization complications. Int J Neurosci. 2022; Apr. 3. doi: 10.1080/00207454.2022.2050907. DOI: 10.1080/00207454.2022.2050907. PMID: 35369847.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Atezolizumab and bevacizumab for hepatocellular carcinoma: How to approach salvage therapy for non-responders?: Editorial on “Sorafenib vs. Lenvatinib in advanced hepatocellular carcinoma after atezolizumab/bevacizumab failure: A real-world study”
- Concurrent transarterial radioembolization and combination atezolizumab/ bevacizumab treatment of infiltrative hepatocellular carcinoma with portal vein tumor thrombosis: a case report
- Complete response in hepatocellular carcinoma with lymph node metastasis by combination therapy of atezolizumab and bevacizumab: a case report
- Favorable response of hepatocellular carcinoma with portal vein tumor thrombosis after radiotherapy combined with atezolizumab plus bevacizumab
- A Case of Acute Transverse Myelitis with Hepatitis B Virus Infection