J Korean Med Sci.
2024 Sep;39(34):e244. 10.3346/jkms.2024.39.e244.
Bevacizumab Alone Versus Bevacizumab Plus Irinotecan in Patients With Recurrent Glioblastoma: A Nationwide Population-Based Study
- Affiliations
-
- 1Department of Neurosurgery, Ajou University School of Medicine, Suwon, Korea
- 2Department of Neurology, McGovern Medical School at UTHealth, Houston, TX, USA
- KMID: 2559199
- DOI: http://doi.org/10.3346/jkms.2024.39.e244
Abstract
- Background
For treating recurrent glioblastoma, for which there is no established treatment, the antiangiogenic antibody, bevacizumab, is used alone or with irinotecan. This study was aimed at comparing the survival of patients with recurrent glioblastoma receiving bevacizumab monotherapy and those receiving bevacizumab plus irinotecan combination therapy (B+I) by using a nationwide population-based dataset.
Methods
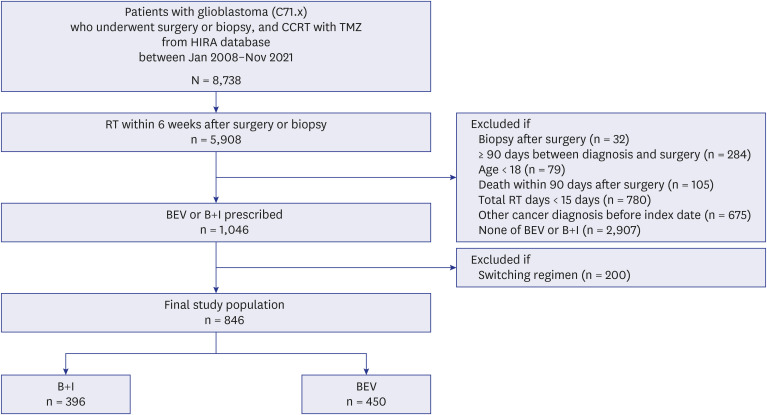
Patients matching the International Classification of Diseases code C71.x were screened from the Health Insurance Review and Assessment Service database. From January 2008 to November 2021, patients who underwent surgery or biopsy and subsequent standard concurrent chemoradiation with temozolomide were included. Among them, those who received bevacizumab monotherapy or B+I were selected. Demographic characteristics, inpatient stay, prescription frequency, survival outcomes, and steroid prescription duration were compared between these two groups.
Results
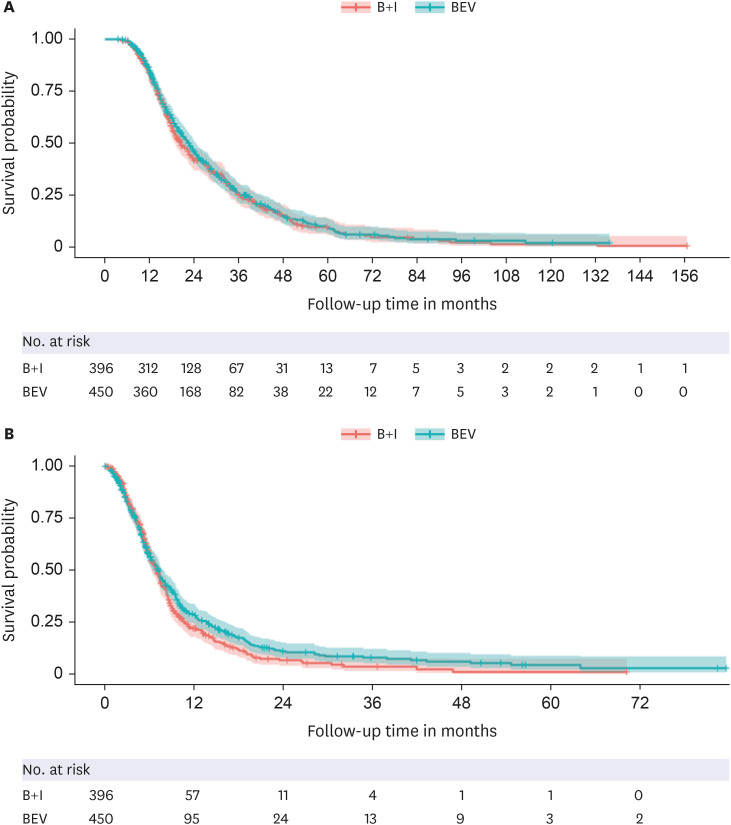
Eight hundred and forty-six patients who underwent surgery or biopsy and received concurrent chemoradiotherapy with temozolomide were included. Of these, 450 and 396 received bevacizumab monotherapy and B+I, respectively. The corresponding median overall survival from the initial surgery was 22.60 months (95% confidence interval [CI], 20.50– 24.21) and 20.44 months (95% CI, 18.55–22.60; P = 0.508, log-rank test). The B+I group had significantly more bevacizumab prescriptions (median 5 times; BEV group: median 3 times). Cox analysis, based on the postsurgery period, revealed that male sex (hazard ratio [HR], 1.28; P = 0.002), older age (HR, 1.01; P = 0.042), and undergoing biopsy instead of surgery (HR, 1.79; P < 0.0001) were significantly associated with decreased survival. Fewer radiotherapy cycles correlated with improved survival outcomes (HR, 0.63; P = 0.001). Cox analysis, conducted from the start of chemotherapy including bevacizumab, showed that male sex was the only variable significantly associated with decreased survival (HR, 1.18; P = 0.044).
Conclusion
We found no significant difference in overall survival between the bevacizumab monotherapy and B+I groups. Considering the additional potential toxicity associated with irinotecan, bevacizumab monotherapy could be a suitable treatment option for treating recurrent glioblastoma.
Figure
Reference
-
1. Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005; 352(10):987–996. PMID: 15758009.2. Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008; 359(5):492–507. PMID: 18669428.3. Tan AC, Ashley DM, López GY, Malinzak M, Friedman HS, Khasraw M. Management of glioblastoma: State of the art and future directions. CA Cancer J Clin. 2020; 70(4):299–312. PMID: 32478924.4. Prajapati HP, Ansari A. Updates in the management of recurrent glioblastoma multiforme. J Neurol Surg. 2022; 30:30.5. Weller M, Cloughesy T, Perry JR, Wick W. Standards of care for treatment of recurrent glioblastoma--are we there yet? Neuro-oncol. 2013; 15(1):4–27. PMID: 23136223.6. Straube C, Kessel KA, Zimmer C, Schmidt-Graf F, Schlegel J, Gempt J, et al. A second course of radiotherapy in patients with recurrent malignant gliomas: clinical data on re-irradiation, prognostic factors, and usefulness of digital biomarkers. Curr Treat Options Oncol. 2019; 20(9):71. PMID: 31324990.7. Buie LW, Valgus J. Bevacizumab: a treatment option for recurrent glioblastoma multiforme. Ann Pharmacother. 2008; 42(10):1486–1490. PMID: 18765835.8. Chahal M, Harrison RA, Thiessen BA. Real-world analysis of outcomes of patients receiving bevacizumab for recurrent glioblastoma in British Columbia. J Clin Oncol. 2022; 40(16 Suppl):e14017.9. Zhang T, Xin Q, Kang JM. Bevacizumab for recurrent glioblastoma: a systematic review and meta-analysis. Eur Rev Med Pharmacol Sci. 2021; 25(21):6480–6491. PMID: 34787852.10. Cloughesy TF, Filka E, Kuhn J, Nelson G, Kabbinavar F, Friedman H, et al. Two studies evaluating irinotecan treatment for recurrent malignant glioma using an every-3-week regimen. Cancer. 2003; 97(9):Suppl. 2381–2386. PMID: 12712460.11. Prados MD, Lamborn K, Yung WK, Jaeckle K, Robins HI, Mehta M, et al. A phase 2 trial of irinotecan (CPT-11) in patients with recurrent malignant glioma: a North American Brain Tumor Consortium study. Neuro-oncol. 2006; 8(2):189–193. PMID: 16533878.12. Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007; 357(26):2666–2676. PMID: 18160686.13. Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006; 355(24):2542–2550. PMID: 17167137.14. Saltz LB, Clarke S, Díaz-Rubio E, Scheithauer W, Figer A, Wong R, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2008; 26(12):2013–2019. PMID: 18421054.15. Ali SA, McHayleh WM, Ahmad A, Sehgal R, Braffet M, Rahman M, et al. Bevacizumab and irinotecan therapy in glioblastoma multiforme: a series of 13 cases. J Neurosurg. 2008; 109(2):268–272. PMID: 18671639.16. Beige A, Ghiringhelli F, Lecuelle J, Truntzer C, Truc G, Vincent J, et al. Efficacy of chemotherapy plus bevacizumab in recurrent glioblastoma multiform: A real-life study. Anticancer Res. 2022; 42(12):5847–5858. PMID: 36456149.17. Bokstein F, Shpigel S, Blumenthal DT. Treatment with bevacizumab and irinotecan for recurrent high-grade glial tumors. Cancer. 2008; 112(10):2267–2273. PMID: 18327820.18. Chamberlain MC. Bevacizumab plus irinotecan in recurrent glioblastoma. J Clin Oncol. 2008; 26(6):1012–1013. PMID: 18281677.19. Desjardins A, Reardon DA, Herndon JE 2nd, Marcello J, Quinn JA, Rich JN, et al. Bevacizumab plus irinotecan in recurrent WHO grade 3 malignant gliomas. Clin Cancer Res. 2008; 14(21):7068–7073. PMID: 18981004.20. Poulsen HS, Grunnet K, Sorensen M, Olsen P, Hasselbalch B, Nelausen K, et al. Bevacizumab plus irinotecan in the treatment patients with progressive recurrent malignant brain tumours. Acta Oncol. 2009; 48(1):52–58. PMID: 19031176.21. Kang TY, Jin T, Elinzano H, Peereboom D. Irinotecan and bevacizumab in progressive primary brain tumors, an evaluation of efficacy and safety. J Neurooncol. 2008; 89(1):113–118. PMID: 18438609.22. Møller S, Grunnet K, Hansen S, Schultz H, Holmberg M, Sorensen M, et al. A phase II trial with bevacizumab and irinotecan for patients with primary brain tumors and progression after standard therapy. Acta Oncol. 2012; 51(6):797–804. PMID: 22548369.23. Park JH, Lee HS, Choi JW, Lim SD, Koh HK, Cho KR, et al. Clinical benefit of bevacizumab and irinotecan (BEV+IRI) in patients with relapsed/refractory glioblastoma (r/rGBM) and its potential predictors. Anticancer Res. 2022; 42(12):6091–6098. PMID: 36456153.24. Vredenburgh JJ, Desjardins A, Herndon JE 2nd, Marcello J, Reardon DA, Quinn JA, et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol. 2007; 25(30):4722–4729. PMID: 17947719.25. Xu T, Chen J, Lu Y, Wolff JE. Effects of bevacizumab plus irinotecan on response and survival in patients with recurrent malignant glioma: a systematic review and survival-gain analysis. BMC Cancer. 2010; 10(1):252. PMID: 20525214.26. Friedman HS, Prados MD, Wen PY, Mikkelsen T, Schiff D, Abrey LE, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009; 27(28):4733–4740. PMID: 19720927.27. Seystahl K, Wiestler B, Hundsberger T, Happold C, Wick W, Weller M, et al. Bevacizumab alone or in combination with irinotecan in recurrent WHO grade II and grade III gliomas. Eur Neurol. 2013; 69(2):95–101. PMID: 23182901.28. Zhang G, Huang S, Wang Z. A meta-analysis of bevacizumab alone and in combination with irinotecan in the treatment of patients with recurrent glioblastoma multiforme. J Clin Neurosci. 2012; 19(12):1636–1640. PMID: 23047061.29. Kreisl TN, Kim L, Moore K, Duic P, Royce C, Stroud I, et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2009; 27(5):740–745. PMID: 19114704.30. Kang H, Song SW, Ha J, Won YJ, Park CK, Yoo H, et al. A Nationwide, population-based epidemiology study of primary central nervous system tumors in Korea, 2007–2016: a comparison with United States data. Cancer Res Treat. 2021; 53(2):355–366. PMID: 33070557.31. Byun YH, Ha J, Kang H, Park CK, Jung KW, Yoo H. Changes in the epidemiologic pattern of primary CNS tumors in response to the aging population: an updated nationwide cancer registry data in the Republic of Korea. JCO Glob Oncol. 2024; 10(10):e2300352. PMID: 38301181.32. Paulík A, Nekvindová J, Filip S. Irinotecan toxicity during treatment of metastatic colorectal cancer: focus on pharmacogenomics and personalized medicine. Tumori. 2020; 106(2):87–94. PMID: 30514181.33. Ahn SV, Lee E, Park B, Jung JH, Park JE, Sheen SS, et al. Cancer development in patients with COPD: a retrospective analysis of the National Health Insurance Service-National Sample Cohort in Korea. BMC Pulm Med. 2020; 20(1):170. PMID: 32539764.34. Lim YC, Lee E, Song J. Depression or anxiety according to management modalities in patients with unruptured intracranial aneurysms. Stroke. 2022; 53(12):3662–3670. PMID: 36128901.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Letter to the Editor: Commentary on “Bevacizumab Alone Versus Bevacizumab Plus Irinotecan in Patients With Recurrent Glioblastoma”
- Recurrent Conjunctival Granuloma Treated with Subconjunctival Bevacizumab
- Bevacizumab in Recurrent Glioma: Patterns of Treatment Failure and Implications
- Post-bevacizumab Clinical Outcomes and the Impact of Early Discontinuation of Bevacizumab in Patients with Recurrent Malignant Glioma
- Clinical Results After Application of Bevacizumab in Recurrent Pterygium