Ann Lab Med.
2024 Sep;44(5):418-425. 10.3343/alm.2023.0325.
Evaluating the TaqMan Jra -Genotyping Method for Rapidly Predicting the Presence of Anti-Jra Antibodies
- Affiliations
-
- 1Department of Laboratory Medicine, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea
- KMID: 2559148
- DOI: http://doi.org/10.3343/alm.2023.0325
Abstract
- Background
The Jr a antigen is a high-prevalence red blood cell (RBC) antigen. Reports on cases of fatal hemolytic disease of the fetus and newborn and acute hemolytic transfusion reactions suggest that antibodies against Jr a (anti-Jra ) have potential clinical significance. Identifying anti-Jra is challenging owing to a lack of commercially available antisera. We developed an alternative approach to rapidly predict the presence of anti-Jra using the TaqMan single-nucleotide polymorphism (SNP)-genotyping method.
Methods
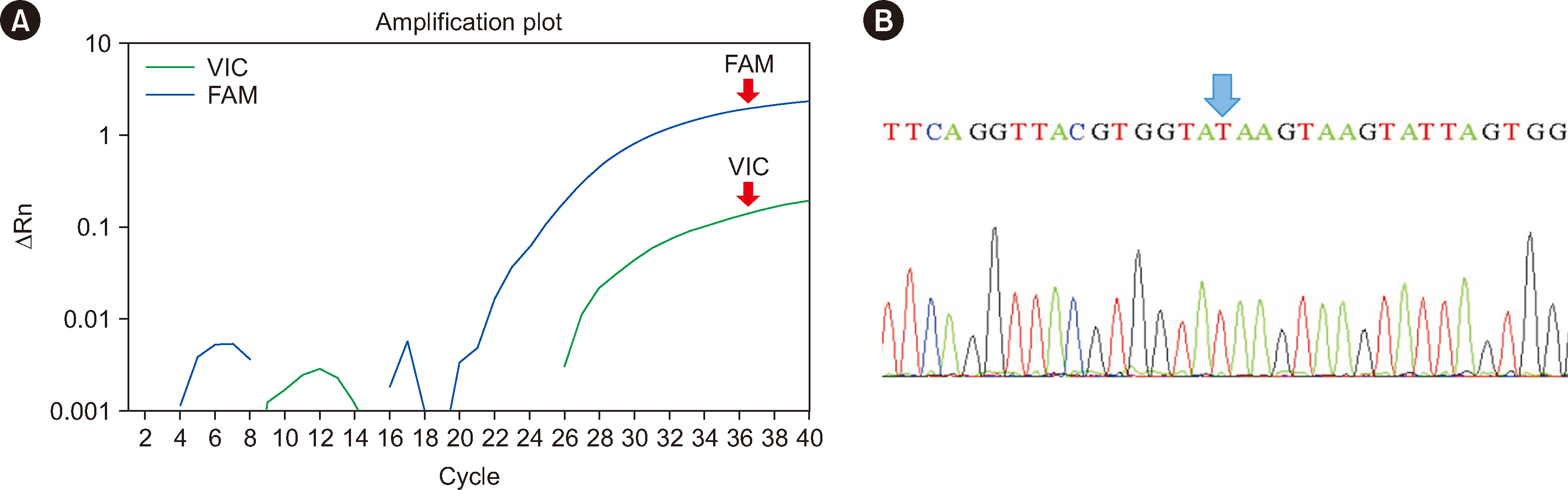
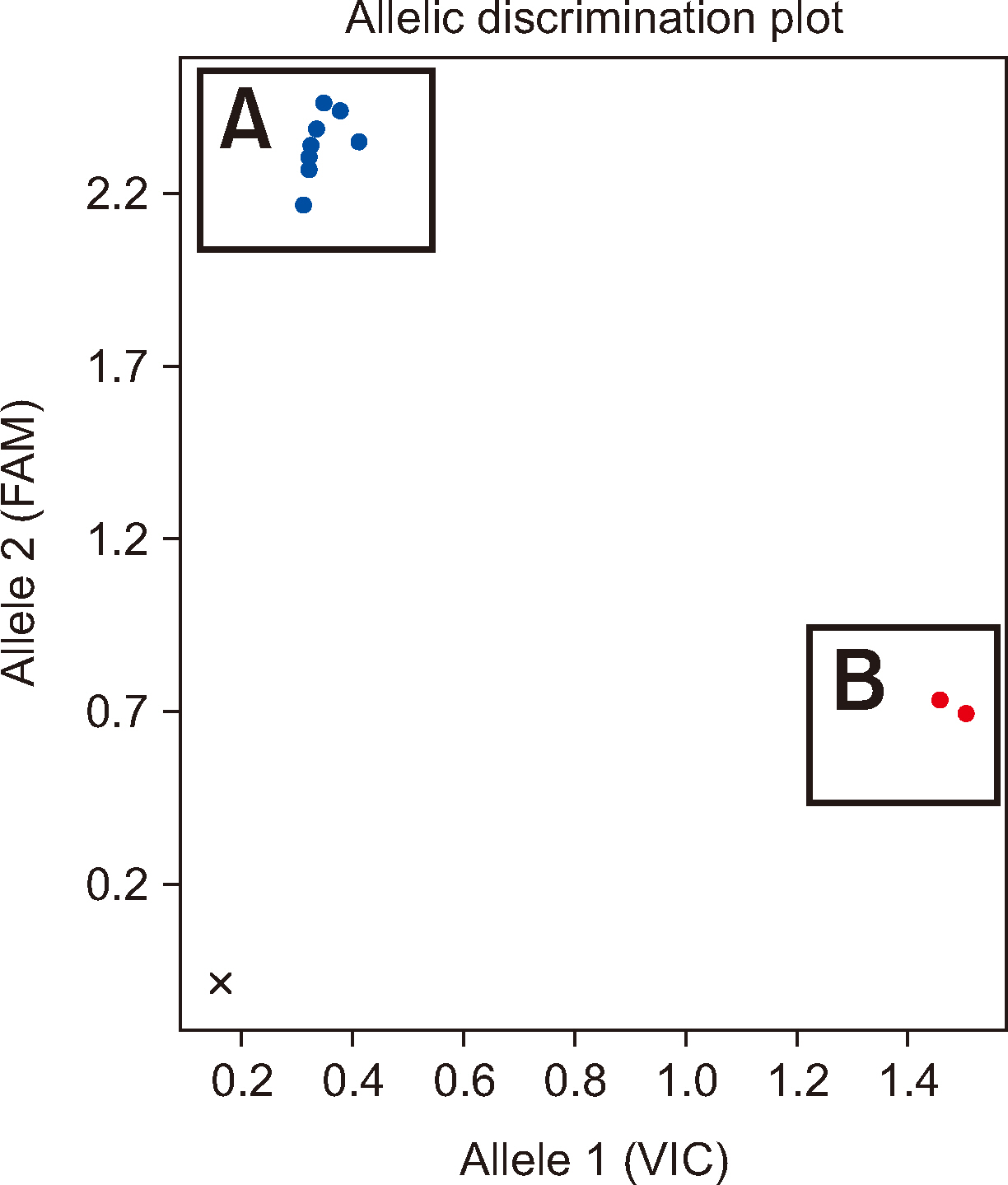
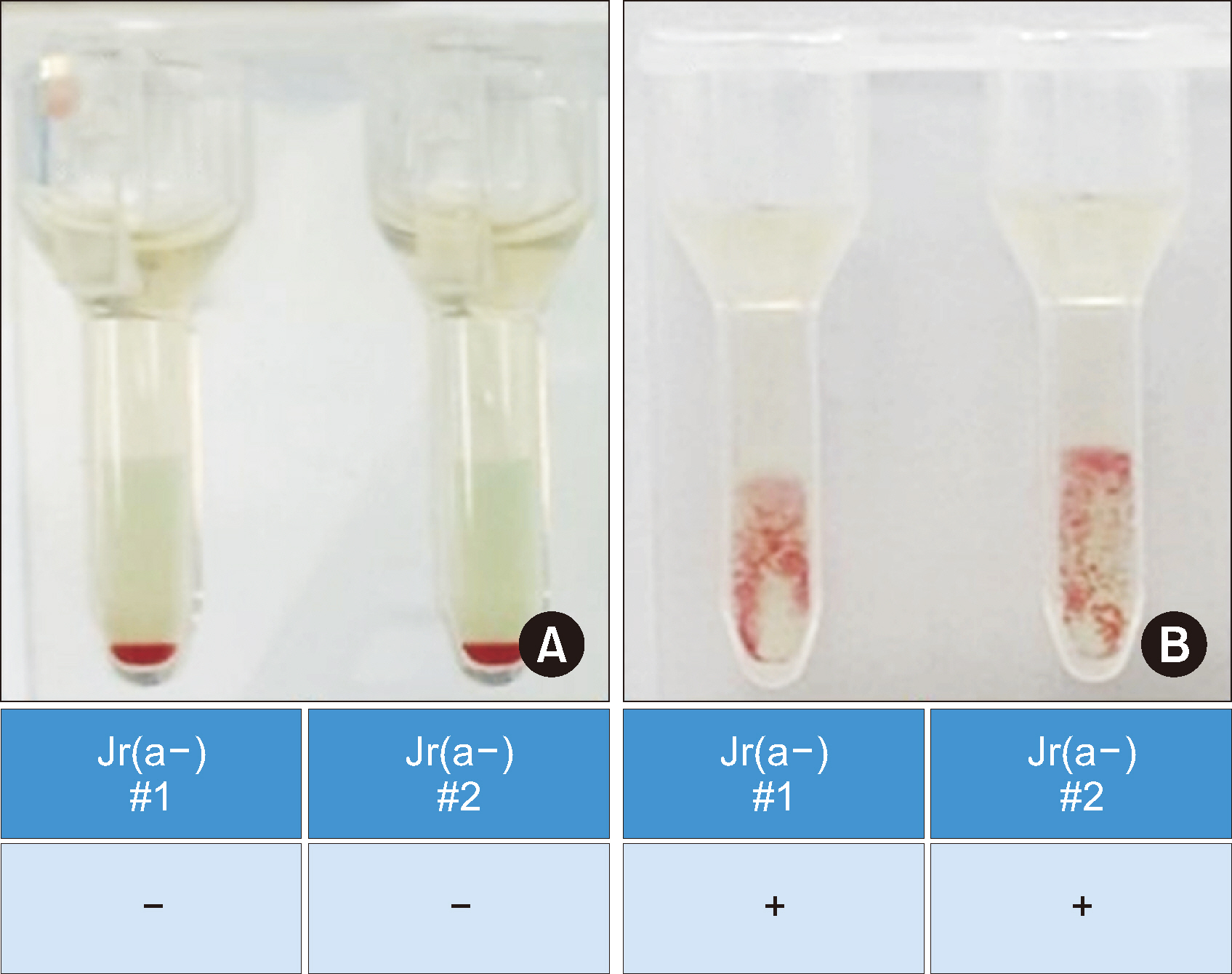
Residual peripheral blood samples from 10 patients suspected of having the anti-Jr a were collected. Two samples with confirmed Jr(a–) RBCs and anti-Jra were used to validate the TaqMan genotyping assay by comparing the genotyping results with direct sequencing. The accuracy of the assay in predicting the presence of anti-Jra was verified through crossmatching with in-house Jr(a–) O+ RBCs.
Results
The TaqMan-genotyping method was validated with two Jr(a–) RBC- and anti-Jra -confirmed samples that showed concordant Jr a genotyping and direct sequencing results. Jra genotyping for the remaining samples and crossmatching the serum samples with inhouse Jr(a–) O+ RBCs showed consistent results.
Conclusions
We validated a rapid, simple, accurate, and cost-effective method for predicting the presence of anti-Jra using a TaqMan-based SNP-genotyping assay. Implementing this method in routine practice in clinical laboratories will assist in solving difficult problems regarding alloantibodies to high-prevalence RBC antigens and ultimately aid in providing safe and timely transfusions and proper patient care.
Keyword
Figure
Reference
-
References
1. Cohn CS, Delaney M, editors. 2020. Technical manual. 20th ed. American Association of Blood Banks;Bethesda: p. 380. DOI: 10.22215/wirl/2020.1.2. Reid ME, Lomas-Francis C. 2012. The blood group antigen facts book. 3rd ed. Academic Press;Cambridge: p. 635–9. DOI: 10.1016/B978-0-12-415849-8.00034-X. PMID: 22258490. PMCID: PMC3677715.3. Zelinski T, Coghlan G, Liu XQ, Reid ME. 2012; ABCG2 null alleles define the Jr(a-) blood group phenotype. Nat Genet. 44:131–2. DOI: 10.1038/ng.1075. PMID: 22246507.
Article4. Saison C, Helias V, Ballif BA, Peyrard T, Puy H, Miyazaki T, et al. 2012; Null alleles of ABCG2 encoding the breast cancer resistance protein define the new blood group system Junior. Nat Genet. 44:174–7. DOI: 10.1038/ng.1070. PMID: 22246505. PMCID: PMC3653631.
Article5. Tritchler JE. 1977; An example of anti-Jra. Transfusion. 17:177–8. DOI: 10.1046/j.1537-2995.1977.17277151926.x. PMID: 850936.6. Kendall AG. 1976; Clinical importance of the rare erythrocyte antibody anti-Jra. Transfusion. 16:646–7. DOI: 10.1046/j.1537-2995.1976.16677060250.x. PMID: 996925.
Article7. Levene C, Sela R, Dvilansky A, Yermiahu T, Daniels G. 1986; The Jr(a-) phenotype and anti-Jra in two Beduin Arab women in Israel. Transfusion. 26:119–20. DOI: 10.1046/j.1537-2995.1986.26186124019.x. PMID: 3946000.
Article8. Vedo M, Reid ME. 1978; Anti-Jra in a Mexican American. Transfusion. 18:569. DOI: 10.1046/j.1537-2995.1978.18579036387.x. PMID: 705864.
Article9. Yamaguchi H, Okubo Y, Seno T, Tomita T, Ogawa Y, Fujii I, et al. 1976; A rare phenotype blood Jr(a-) occurring in two successive generations of a Japanese family. Proc Jpn Acad. 52:521–3. DOI: 10.2183/pjab1945.52.521.
Article10. Nakajima H, Ito K. 1978; An example of anti-Jra causing hemolytic disease of the newborn and frequency of Jra antigen in the Japanese population. Vox Sang. 35:265–7. DOI: 10.1111/j.1423-0410.1978.tb02932.x. PMID: 567888.
Article11. Okubo Y. 1978; Some rare blood group phenotypes in Japanese, Fy (a-), Di (b-) and Jr (a-). Blood Programme. 1:279–84. DOI: 10.5924/abgri1972.1978.1.12. Castilho L, Reid ME. 2013; A review of the JR blood group system. Immunohematology. 29:63–8. DOI: 10.21307/immunohematology-2019-126. PMID: 24094238.
Article13. Stroup M, MacIlroy M. Five examples of an antibody defining an antigen of high frequency in the Caucasian population. Proceedings of the 23rd Annual Meeting of the American Association of Blood Banks. 1970; San Francisco. p. 86.14. Peyrard T, Pham BN, Arnaud L, Fleutiaux S, Brossard Y, Guerin B, et al. 2008; Fatal hemolytic disease of the fetus and newborn associated with anti-Jr. Transfusion. 48:1906–11. DOI: 10.1111/j.1537-2995.2008.01787.x. PMID: 18522708.
Article15. Arriaga F, Gomez I, Linares MD, Gascon A, Carpio N, Perales A. 2009; Fatal hemolytic disease of the fetus and newborn possibly due to anti-Jr. Transfusion. 49:813. DOI: 10.1111/j.1537-2995.2008.02087.x. PMID: 19335378.
Article16. Kim MS, Kim JS, Park H, Chung Y, Kim H, Ko DH, et al. 2020; Fatal hemolytic disease of the fetus and newborn caused by anti-Jra antibody: a case report and literature review. Transfus Apher Sci. 59:102605. DOI: 10.1016/j.transci.2019.06.029. PMID: 31324575.17. Kwon MY, Su L, Arndt PA, Garratty G, Blackall DP. 2004; Clinical significance of anti-Jra: report of two cases and review of the literature. Transfusion. 44:197–201. DOI: 10.1111/j.1537-2995.2004.00643.x. PMID: 14962310.
Article18. Choi SJ, Chung YN, Cho D, Kim S. 2019; Alloantibodies to high-incidence antigen: review of cases and transfusion experiences in Korea. Korean J Blood Transfus. 30:101–12. DOI: 10.17945/kjbt.2019.30.2.101.
Article19. International Society of Blood Transfusion. Names for JR (ISBT 032) blood group alleles. https://www.isbtweb.org/resource/032jr.html. Updated on July 2023.20. Haer-Wigman L, Ait Soussan A, Ligthart P, de Haas M, van der Schoot CE. 2014; Molecular analysis of immunized Jr(a-) or Lan- patients and validation of a high-throughput genotyping assay to screen blood donors for Jr(a-) and Lan- phenotypes. Transfusion. 54:1836–46. DOI: 10.1111/trf.12544. PMID: 24456066.21. Hue-Roye K, Lomas-Francis C, Coghlan G, Zelinski T, Reid ME. 2013; The JR blood group system (ISBT 032): molecular characterization of three new null alleles. Transfusion. 53:1575–9. DOI: 10.1111/j.1537-2995.2012.03930.x. PMID: 23066723.22. Karczewski KJ, Francioli LC, Tiao G, Cummings BB, Alföldi J, Wang Q, et al. 2020; The mutational constraint spectrum quantified from variation in 141,456 humans. Nature. 581:434–43. DOI: 10.1038/s41586-020-2308-7. PMID: 32461654. PMCID: PMC7334197.
Article23. Jung KS, Hong KW, Jo HY, Choi J, Ban HJ, Cho SB, et al. 2020; KRGDB: the large-scale variant database of 1722 Koreans based on whole genome sequencing. Database (Oxford). 2020:baz146. DOI: 10.1093/database/baz146. PMID: 32133509. PMCID: PMC7056612.
Article24. Cho YK, Kim S, Lim J, Ko DH, Choi SJ, Kim HO. 2022; Development of cryopreserved red blood cell panels as biological reference standards for performance evaluation of ABO and D blood grouping reagents. Lab Med Online. 12:1–10. DOI: 10.47429/lmo.2022.12.1.1.
Article25. Song S, Choi J, Kim S, Kim HO, Min H, Kim J, et al. 2009; Development of cryopreserved red blood cell panels for verifying ABO and D blood grouping reagents. Korean J Blood Transfus. 20:46–54.26. Meryman HT, Hornblower M. 1972; A method for freezing and washing red blood cells using a high glycerol concentration. Transfusion. 12:145–56. DOI: 10.1111/j.1537-2995.1972.tb00001.x. PMID: 5026166.
Article27. Högman CF, Hornblower MLS, Flodin M, Gillberg G, Meryman HT, Säfwenberg J. 1986; A simple method for high-quality frozen red cells in blood group serology. Transfusion. 26:434–6. DOI: 10.1046/j.1537-2995.1986.26587020120.x. PMID: 3765037.
Article28. Lelkens CCM, Noorman F, Koning JG, Truijens-de Lange R, Stekkinger PS, Bakker JC, et al. 2003; Stability after thawing of RBCs frozen with the high-and low-glycerol method. Transfusion. 43:157–64. DOI: 10.1046/j.1537-2995.2003.00293.x. PMID: 12559010.29. Yun JW, Kang ES, Ki CS, Koh KC, Kim DW. 2012; Sensitization to multiple rh antigens by transfusion of random donor platelet concentrates in a -D- phenotype patient. Ann Lab Med. 32:429–32. DOI: 10.3343/alm.2012.32.6.429. PMID: 23130343. PMCID: PMC3486938.
Article30. Orzińska A. 2023; Next generation sequencing and blood group genotyping: a narrative review. Ann Blood. 8:4. DOI: 10.21037/aob-21-39.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- JR Blood Group and Anti-Jra Antibody
- A Case Report of Anti-Jr(a) in Pregnant Woman
- Hemolytic Disease of the Newborn Associated with Anti-Jr(a) Alloimmunization in a Twin Pregnancy: The First Case Report in Korea
- Comparison of Two Real-Time PCR Assays for Apolipoprotein E Genotyping
- Commentary on “Navigation-Guided/Robot-Assisted Spinal Surgery: A Review Article”