J Pathol Transl Med.
2024 Sep;58(5):205-213. 10.4132/jptm.2024.06.24.
Educational exchange in thyroid core needle biopsy diagnosis: enhancing pathological interpretation through guideline integration and peer learning
- Affiliations
-
- 1Department of Anatomical Pathology, Faculty of Medicine Universitas Indonesia - Dr. Cipto Mangunkusumo Hospital, Jakarta, Indonesia
- 2Department of Hospital Pathology, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 3Cancer Research Institute, College of Medicine, The Catholic University of Korea, Seoul, Korea
- KMID: 2559065
- DOI: http://doi.org/10.4132/jptm.2024.06.24
Abstract
- Background
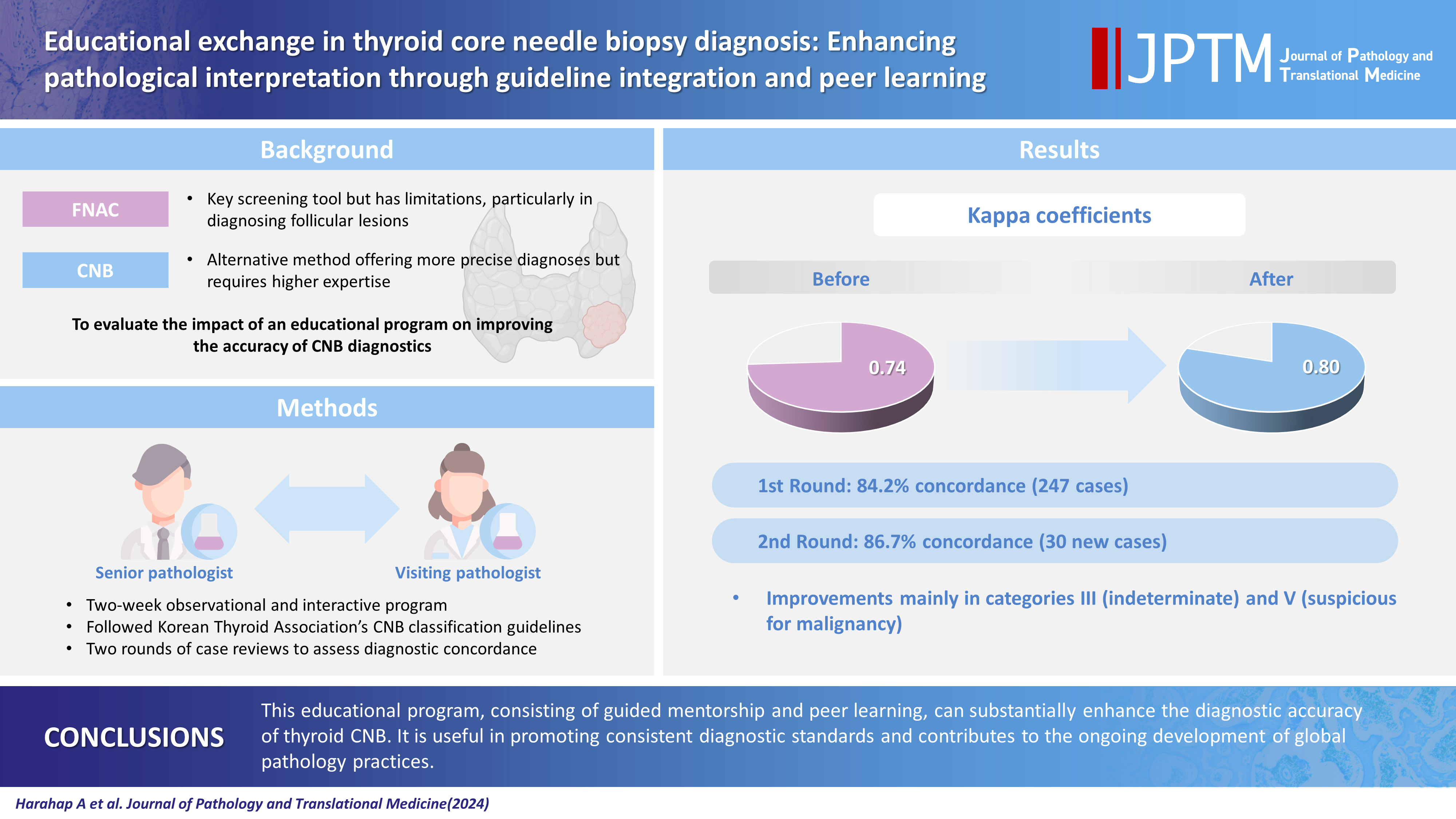
While fine needle aspiration cytology (FNAC) plays an essential role in the screening of thyroid nodules, core needle biopsy (CNB) acts as an alternative method to address FNAC limitations. However, diagnosing thyroid CNB samples can be challenging due to variations in background and levels of experience. Effective training is indispensable to mitigate this challenge. We aim to evaluate the impact of an educational program on improving the accuracy of CNB diagnostics.
Methods
The 2-week observational program included a host mentor pathologist with extensive experience and a visiting pathologist. The CNB classification by The Practice Guidelines Committee of the Korean Thyroid Association was used for the report. Two rounds of reviewing the case were carried out, and the level of agreement between the reviewers was analyzed.
Results
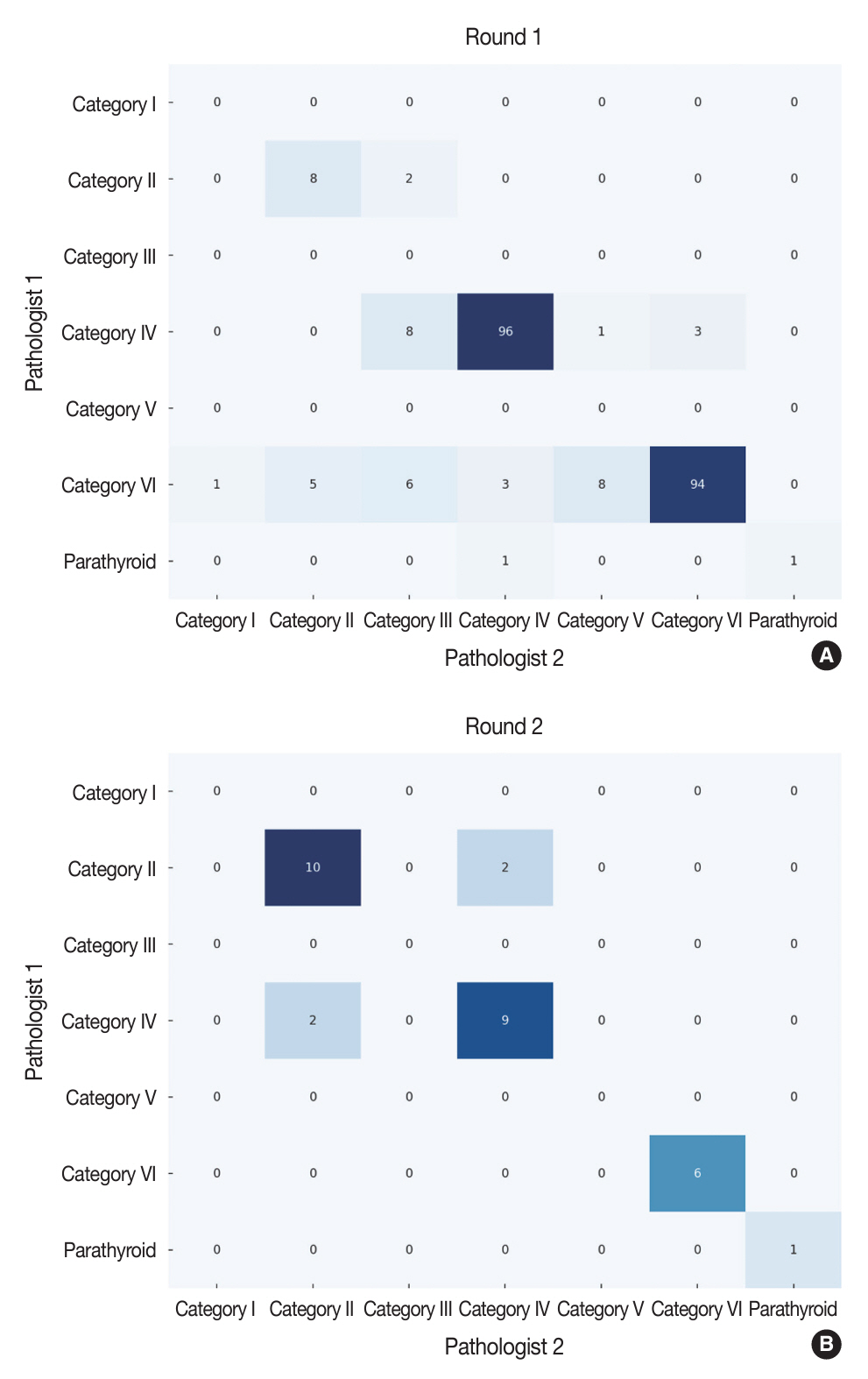
The first-round assessment showed a concordance between two pathologists for 247 thyroid CNB specimens by 84.2%, with a kappa coefficient of 0.74 (indicating substantial agreement). This finding was attributed to the discordance in the use of categories III and V. After peer learning, the two pathologists evaluated 30 new cases, which showed an overall improvement in the level of agreement. The percentage of agreement between pathologists on thyroid CNB diagnosis was 86.7%, as measured by kappa coefficient of 0.80.
Conclusions
This educational program, consisting of guided mentorship and peer learning, can substantially enhance the diagnostic accuracy of thyroid CNB. It is useful in promoting consistent diagnostic standards and contributes to the ongoing development of global pathology practices.
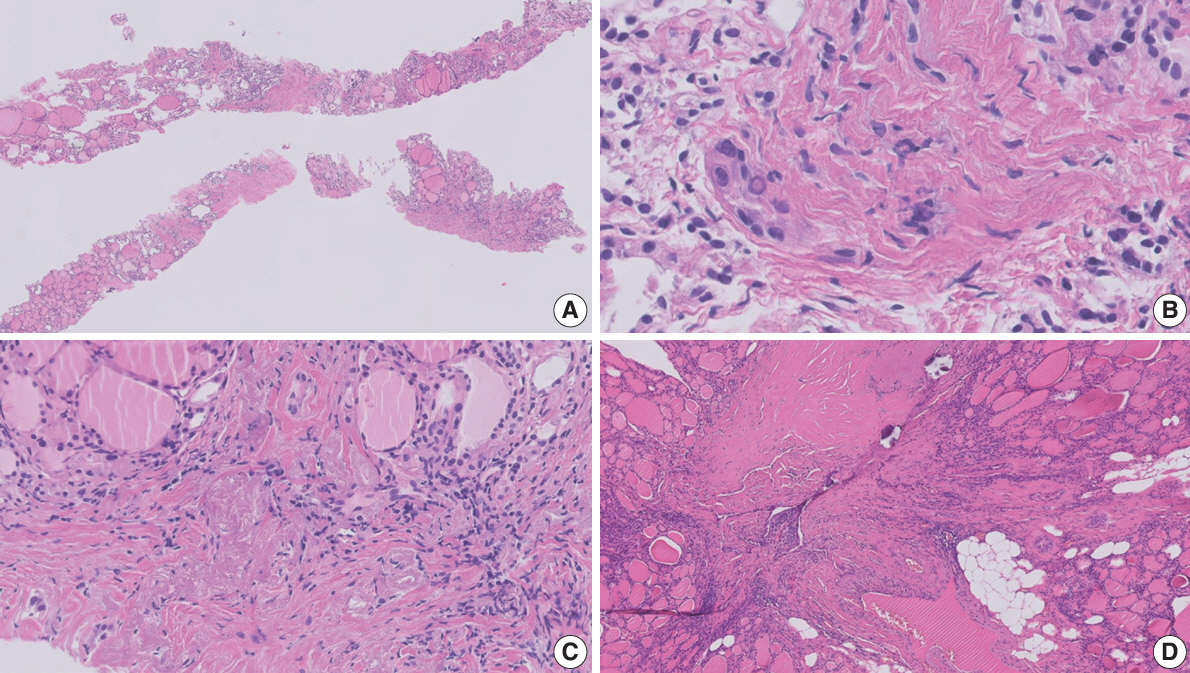
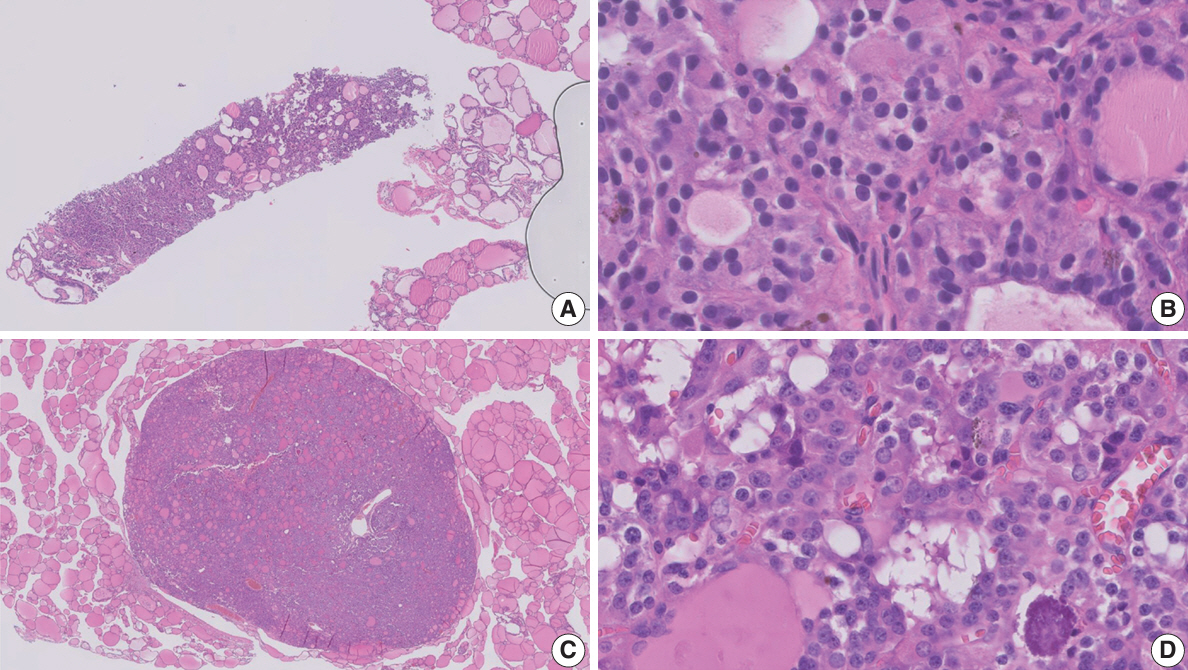
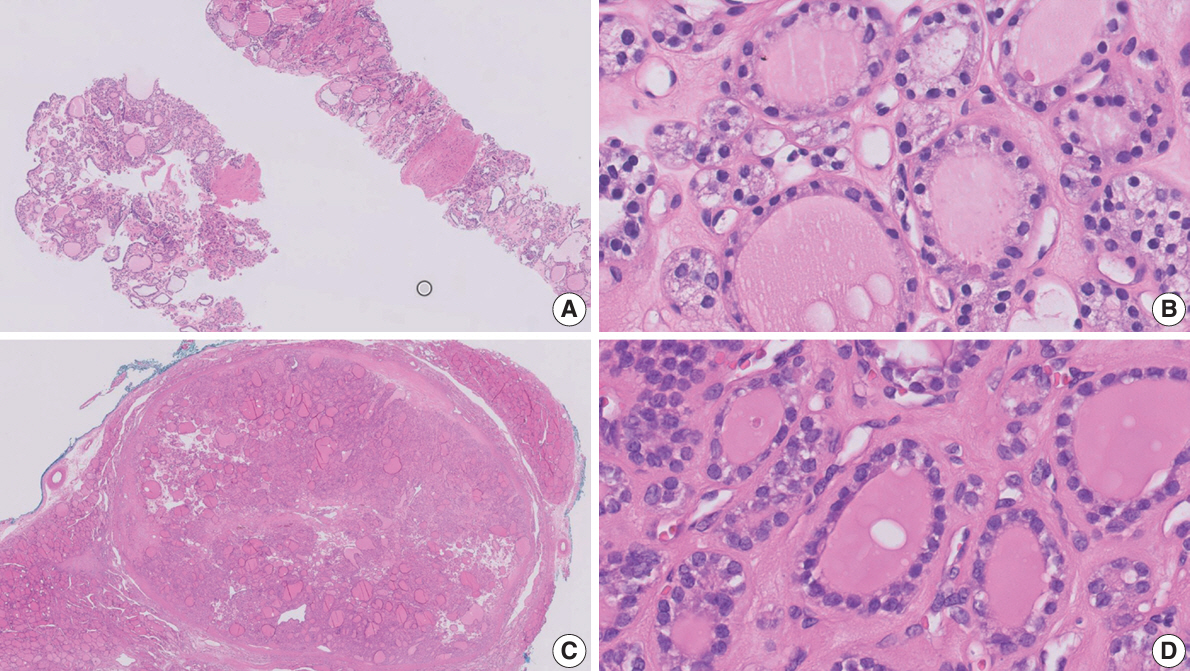
Figure
Reference
-
References
1. Kitahara CM, Schneider AB. Epidemiology of thyroid cancer. Cancer Epidemiol Biomarkers Prev. 2022; 31:1284–97.2. Lee EK, Park YJ, Jung CK, Na DG. A narrative review of the 2023 Korean Thyroid Association management guideline for patients with thyroid nodules. Endocrinol Metab (Seoul). 2024; 39:61–72.3. Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016; 26:1–133.4. Ali SZ, Baloch ZW, Cochand-Priollet B, Schmitt FC, Vielh P, VanderLaan PA. The 2023 Bethesda System for Reporting Thyroid Cytopathology. Thyroid. 2023; 33:1039–44.5. Doubi A, Alrayes NS, Alqubaisi AK, Al-Dhahri SF. The value of repeating fine-needle aspiration for thyroid nodules. Ann Saudi Med. 2021; 41:36–42.6. Suh CH, Baek JH, Choi YJ, et al. Efficacy and safety of core-needle biopsy in initially detected thyroid nodules via propensity score analysis. Sci Rep. 2017; 7:8242.7. Kim HC, Kim YJ, Han HY, et al. First-line use of core needle biopsy for high-yield preliminary diagnosis of thyroid nodules. AJNR Am J Neuroradiol. 2017; 38:357–63.8. Paja M, Del Cura JL, Zabala R, Korta I, Ugalde A, Lopez JI. Coreneedle biopsy in thyroid nodules: performance, accuracy, and complications. Eur Radiol. 2019; 29:4889–96.9. Jung CK, Baek JH, Na DG, Oh YL, Yi KH, Kang HC. 2019 Practice guidelines for thyroid core needle biopsy: a report of the Clinical Practice Guidelines Development Committee of the Korean Thyroid Association. J Pathol Transl Med. 2020; 54:64–86.10. Hahn SY, Shin JH, Oh YL, Park KW. Ultrasound-guided core needle biopsy techniques for intermediate or low suspicion thyroid nodules: which method is effective for diagnosis? Korean J Radiol. 2019; 20:1454–61.11. Suh CH, Baek JH, Lee JH, et al. The role of core-needle biopsy in the diagnosis of thyroid malignancy in 4580 patients with 4746 thyroid nodules: a systematic review and meta-analysis. Endocrine. 2016; 54:315–28.12. Padmanabhan V, Marshall CB, Akdas Barkan G, et al. Reproducibility of atypia of undetermined significance/follicular lesion of undetermined significance category using the bethesda system for reporting thyroid cytology when reviewing slides from different institutions: a study of interobserver variability among cytopathologists. Diagn Cytopathol. 2017; 45:399–405.13. Clary KM, Condel JL, Liu Y, Johnson DR, Grzybicki DM, Raab SS. Interobserver variability in the fine needle aspiration biopsy diagnosis of follicular lesions of the thyroid gland. Acta Cytol. 2005; 49:378–82.14. Sripodok S, Benjakul N. Interobserver variability in inconclusive diagnostic categories of thyroid fine needle aspiration cytology: an urban-based tertiary hospital experience. Ann Diagn Pathol. 2023; 63:152083.15. Jung CK. Reevaluating diagnostic categories and associated malignancy risks in thyroid core needle biopsy. J Pathol Transl Med. 2023; 57:208–16.16. Elsheikh TM, Asa SL, Chan JK, et al. Interobserver and intraobserver variation among experts in the diagnosis of thyroid follicular lesions with borderline nuclear features of papillary carcinoma. Am J Clin Pathol. 2008; 130:736–44.17. Seok JY, An J, Cho HY, Kim Y, Ha SY. Nuclear features of papillary thyroid carcinoma: comparison of core needle biopsy and thyroidectomy specimens. Ann Diagn Pathol. 2018; 32:35–40.18. Haq F, Bychkov A, Jung CK. A matched-pair analysis of nuclear morphologic features between core needle biopsy and surgical specimen in thyroid tumors using a deep learning model. Endocr Pathol. 2022; 33:472–83.19. Baloch ZW, Asa SL, Barletta JA, et al. Overview of the 2022 WHO classification of thyroid neoplasms. Endocr Pathol. 2022; 33:27–63.20. Jung CK, Bychkov A, Kakudo K. Update from the 2022 World Health Organization classification of thyroid tumors: a standardized diagnostic approach. Endocrinol Metab (Seoul). 2022; 37:703–18.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Surgical Management of Bleeding from the Superior Thyroid Artery after Core Needle Biopsy
- Effectiveness and Limitations of Core Needle Biopsy in the Diagnosis of Thyroid Nodules: Review of Current Literature
- RE: Thyroid Core Needle Biopsy: The Strengths of Guidelines of the Korean Society of Thyroid Radiology
- Thyroid Nodules with Nondiagnostic FNA Results: Role of Core Needle Biopsy
- Recent Advances in Core Needle Biopsy for Thyroid Nodules