Diabetes Metab J.
2024 Sep;48(5):929-936. 10.4093/dmj.2023.0297.
Efficacy and Safety of IDegAsp in a Real-World Korean Population with Type 2 Diabetes Mellitus
- Affiliations
-
- 1Division of Endocrinology, Department of Internal Medicine, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Korea
- 2Division of Endocrinology and Metabolism, Department of Internal Medicine, St. Vincent’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 3Department of Internal Medicine, Chungbuk National University Hospital, Chungbuk National University College of Medicine, Cheongju, Korea
- 4Division of Endocrinology and Metabolism, Department of Internal Medicine, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea
- 5Division of Endocrinology and Metabolism, Department of Internal Medicine, Soonchunhyang University Cheonan Hospital, Soonchunhyang University College of Medicine, Cheonan, Korea
- 6Novo Nordisk Pharma Korea Limited, Seoul, Korea
- 7Novo Nordisk Region Asia Pacific, Dubai, United Arab Emirates
- 8Department of Internal Medicine, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea
- KMID: 2559002
- DOI: http://doi.org/10.4093/dmj.2023.0297
Abstract
- Background
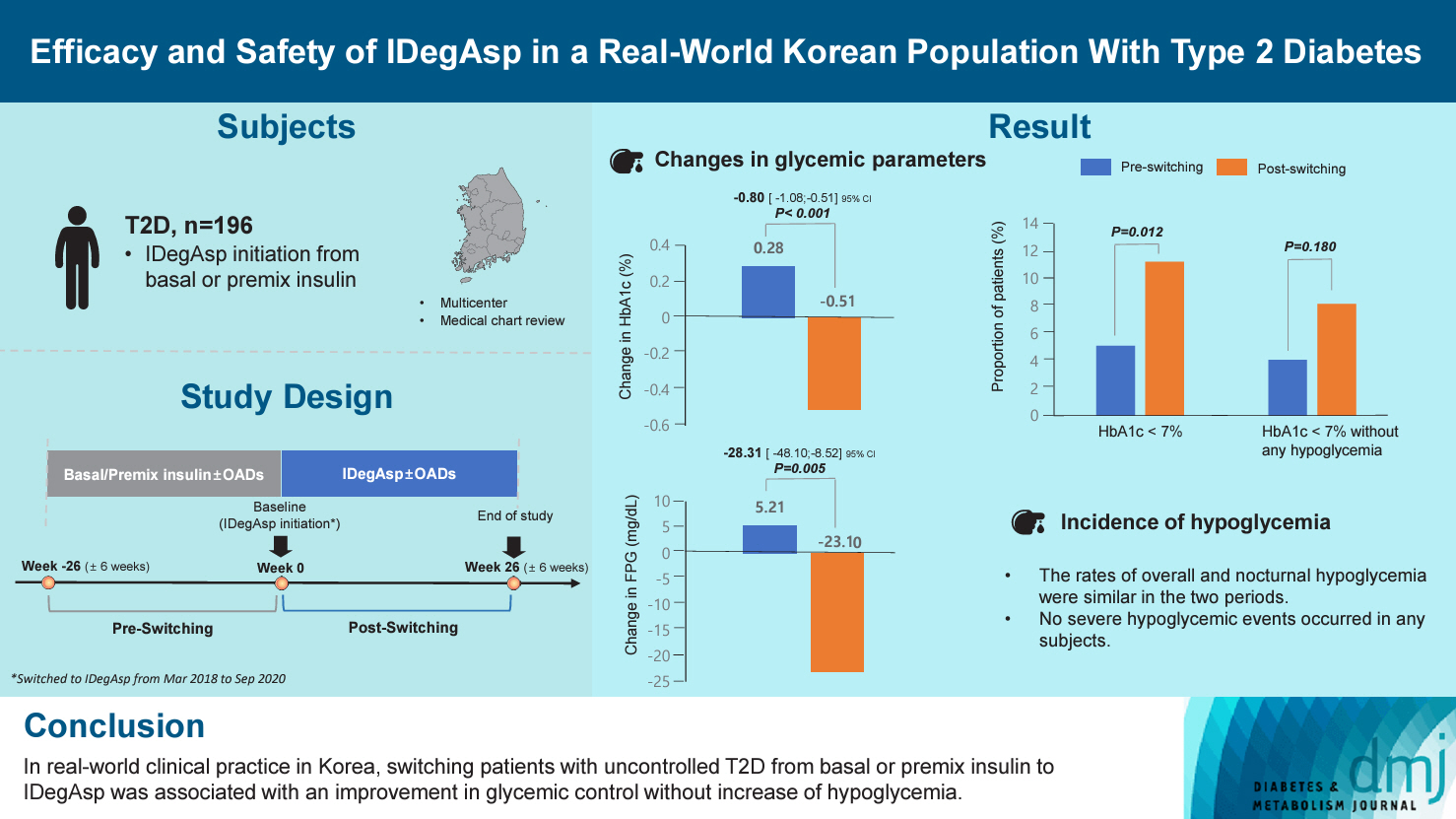
This study investigated the real-world efficacy and safety of insulin degludec/insulin aspart (IDegAsp) in Korean adults with type 2 diabetes mellitus (T2DM), whose insulin treatment was switched to IDegAsp.
Methods
This was a multicenter, retrospective, observational study comprising two 26-week treatment periods, before and after switching to IDegAsp, respectively. Korean adults with uncontrolled T2DM treated with basal or premix insulin (±oral antidiabetic drugs) were enrolled. The primary objective was to compare the degree of glycosylated hemoglobin (HbA1c) change in each 26-week observation period. The analyses included changes in HbA1c, fasting plasma glucose (FPG), body weight, proportion of participants achieving HbA1c <7.0%, hypoglycemic events, and total daily insulin dose (ClinicalTrials.gov, number NCT04656106).
Results
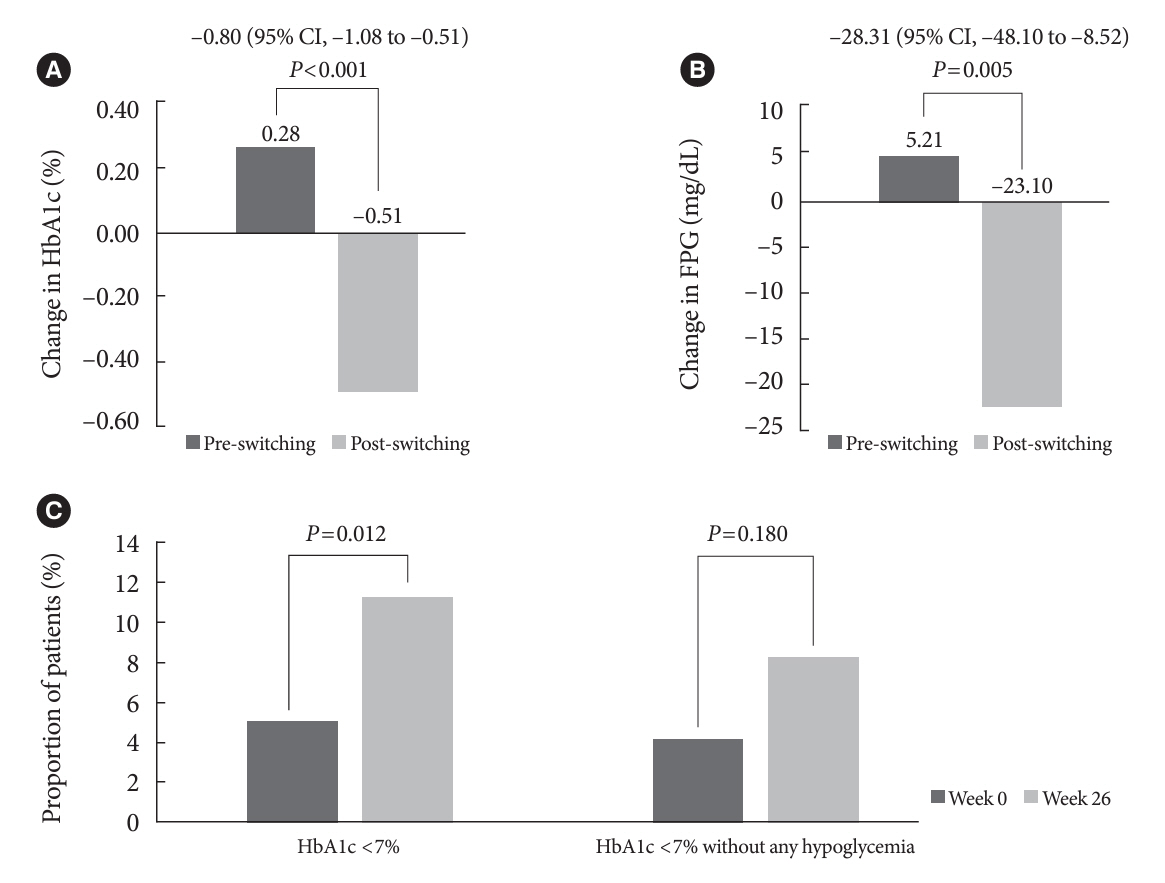
In total, 196 adults (mean age, 65.95 years; mean T2DM duration, 18.99 years) were analyzed. The change in both HbA1c and FPG were significantly different between the pre-switching and the post-switching period (0.28% vs. –0.51%, P<0.001; 5.21 mg/dL vs. –23.10 mg/dL, P=0.005), respectively. After switching, the rate of achieving HbA1c <7.0% was significantly improved (5.10% at baseline vs. 11.22% with IDegAsp, P=0.012). No significant differences (before vs. after switching) were observed in body weight change, and total daily insulin dose. The rates of overall and severe hypoglycemia were similar in the two periods.
Conclusion
In real-world clinical practice in Korea, the change of insulin regimen to IDegAsp was associated with an improvement in glycemic control without increase of hypoglycemia, supporting the use of IDegAsp for patients with T2DM uncontrolled with basal or premix insulin.
Keyword
Figure
Reference
-
1. Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003; 52:102–10.2. Bae JH, Han KD, Ko SH, Yang YS, Choi JH, Choi KM, et al. Diabetes fact sheet in Korea 2021. Diabetes Metab J. 2022; 46:417–26.
Article3. Organisation for Economic Co-operation and Development. OECD Health Statistics 2022. Available from: https://stats.oecd.org/Index.aspx?QueryId=30115 (cited 2024 Jan 16).4. Ko SH. 2021 Clinical practice guidelines for diabetes mellitus in Korea. J Korean Diabetes. 2021; 22:244–9.
Article5. American Diabetes Association Professional Practice Committee. 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes-2022. Diabetes Care. 2022; 45(Suppl 1):S125–43.6. Rodbard HW, Cariou B, Zinman B, Handelsman Y, PhilisTsimikas A, Skjoth TV, et al. Comparison of insulin degludec with insulin glargine in insulin-naive subjects with type 2 diabetes: a 2-year randomized, treat-to-target trial. Diabet Med. 2013; 30:1298–304.7. Zinman B, Philis-Tsimikas A, Cariou B, Handelsman Y, Rodbard HW, Johansen T, et al. Insulin degludec versus insulin glargine in insulin-naive patients with type 2 diabetes: a 1-year, randomized, treat-to-target trial (BEGIN Once Long). Diabetes Care. 2012; 35:2464–71.8. Haahr H, Fita EG, Heise T. A review of insulin degludec/insulin aspart: pharmacokinetic and pharmacodynamic properties and their implications in clinical use. Clin Pharmacokinet. 2017; 56:339–54.
Article9. Novo Nordisk A/S. Ryzodeg summary of product characteristics. Available from: https://www.ema.europa.eu/en/documents/product-information/ryzodeg-epar-product-information_en.pdf (cited 2024 Jan 16).10. Franek E, Haluzik M, Canecki Varzic S, Sargin M, Macura S, Zacho J, et al. Twice-daily insulin degludec/insulin aspart provides superior fasting plasma glucose control and a reduced rate of hypoglycaemia compared with biphasic insulin aspart 30 in insulin-naïve adults with type 2 diabetes. Diabet Med. 2016; 33:497–505.
Article11. Fulcher GR, Christiansen JS, Bantwal G, Polaszewska-Muszynska M, Mersebach H, Andersen TH, et al. Comparison of insulin degludec/insulin aspart and biphasic insulin aspart 30 in uncontrolled, insulin-treated type 2 diabetes: a phase 3a, randomized, treat-to-target trial. Diabetes Care. 2014; 37:2084–90.
Article12. Kaneko S, Chow F, Choi DS, Taneda S, Hirao K, Park Y, et al. Insulin degludec/insulin aspart versus biphasic insulin aspart 30 in Asian patients with type 2 diabetes inadequately controlled on basal or pre-/self-mixed insulin: a 26-week, randomised, treat-to-target trial. Diabetes Res Clin Pract. 2015; 107:139–47.
Article13. Niskanen L, Leiter LA, Franek E, Weng J, Damci T, Munoz-Torres M, et al. Comparison of a soluble co-formulation of insulin degludec/insulin aspart vs biphasic insulin aspart 30 in type 2 diabetes: a randomised trial. Eur J Endocrinol. 2012; 167:287–94.
Article14. Yang W, Ma J, Hong T, Liu M, Miao H, Peng Y, et al. Efficacy and safety of insulin degludec/insulin aspart versus biphasic insulin aspart 30 in Chinese adults with type 2 diabetes: a phase III, open-label, 2:1 randomized, treat-to-target trial. Diabetes Obes Metab. 2019; 21:1652–60.
Article15. Fulcher GR, Akhtar S, Al-Jaser SJ, Medina J, Mohamed M, Nicodemus NA Jr, et al. Initiating or switching to insulin degludec/insulin aspart in adults with type 2 diabetes: a real-world, prospective, non-interventional study across six countries. Adv Ther. 2022; 39:3735–48.
Article16. World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013; 310:2191–4.17. Jang HN, Yang YS, Lee SO, Oh TJ, Koo BK, Jung HS. Favorable glycemic control with once-daily insulin degludec/insulin aspart after changing from basal insulin in adults with type 2 diabetes. Endocrinol Metab (Seoul). 2019; 34:382–9.
Article18. Jang HN, Yang YS, Oh TJ, Koo BK, Lee SO, Park KS, et al. Low fasting glucose-to-estimated average glucose ratio was associated with superior response to insulin degludec/aspart compared with basal insulin in patients with type2 diabetes. J Diabetes Investig. 2022; 13:85–93.
Article19. Shigiyama F, Liu L, Nordahl H, Suzuki R, Yamamoto Y, Hirose T. A real-world, prospective, non-interventional study of adults with T2D switching to IDegAsp from glargine U100 or U300 in Japan. Diabetes Ther. 2021; 12:2405–21.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Switching to Once-Daily Insulin Degludec/Insulin Aspart from Basal Insulin Improves Postprandial Glycemia in Patients with Type 2 Diabetes Mellitus: Randomized Controlled Trial
- Clinical Efficacy of Sodium-Glucose Cotransporter 2 Inhibitor and Glucagon-Like Peptide-1 Receptor Agonist Combination Therapy in Type 2 Diabetes Mellitus: Real-World Study (Diabetes Metab J 2022;46: 658-62)
- Clinical Efficacy of Sodium-Glucose Cotransporter 2 Inhibitor and Glucagon-Like Peptide-1 Receptor Agonist Combination Therapy in Type 2 Diabetes Mellitus: Real-World Study
- Pharmacothearpy of Adolescents with Diabetes
- Safety of COVID-19 Vaccines among Patients with Type 2 Diabetes Mellitus: Real-World Data Analysis (Diabetes Metab J 2023;47:356-65)