Endocrinol Metab.
2024 Aug;39(4):559-568. 10.3803/EnM.2024.2025.
Regulation of Energy and Glucose Homeostasis by the Nucleus of the Solitary Tract and the Area Postrema
- Affiliations
-
- 1Institute of Medical Science, University of Toronto, Toronto, ON, Canada
- 2Toronto General Hospital Research Institute, University Health Network (UHN), Toronto, ON, Canada
- 3Department of Physiology, University of Toronto, Toronto, ON, Canada
- 4Department of Medicine, Medicine, University of Toronto, Toronto, ON, Canada
- 5Banting and Best Diabetes Center, University of Toronto, Toronto, ON, Canada
- KMID: 2558938
- DOI: http://doi.org/10.3803/EnM.2024.2025
Abstract
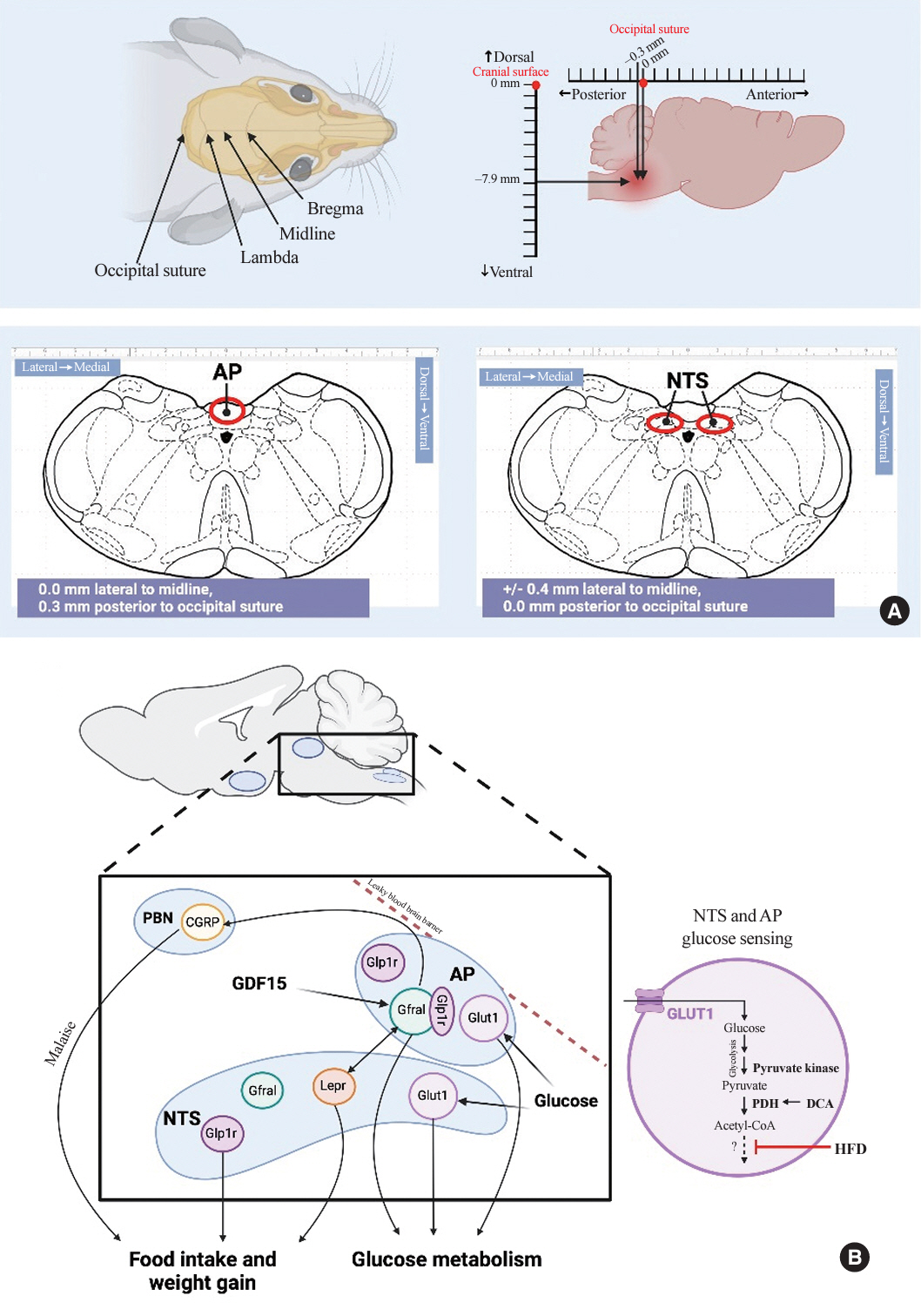
- The central nervous system regulates feeding, weight and glucose homeostasis in rodents and humans, but the site-specific mechanisms remain unclear. The dorsal vagal complex in the brainstem that contains the nucleus of the solitary tract (NTS) and area postrema (AP) emerges as a regulatory center that impacts energy and glucose balance by monitoring hormonal and nutrient changes. However, the specific mechanistic metabolic roles of the NTS and AP remain elusive. This mini-review highlights methods to study their distinct roles and recent findings on their metabolic differences and similarities of growth differentiation factor 15 (GDF15) action and glucose sensing in the NTS and AP. In summary, future research aims to characterize hormonal and glucose sensing mechanisms in the AP and/or NTS carries potential to unveil novel targets that lower weight and glucose levels in obesity and diabetes.
Keyword
Figure
Reference
-
1. Myers MG Jr, Olson DP. Central nervous system control of metabolism. Nature. 2012; 491:357–63.
Article2. Woods SC. The control of food intake: behavioral versus molecular perspectives. Cell Metab. 2009; 9:489–98.
Article3. Deem JD, Tingley D, Bjerregaard AM, Secher A, Chan O, Uzo C, et al. Identification of hypothalamic glucoregulatory neurons that sense and respond to changes in glycemia. Diabetes. 2023; 72:1207–13.4. Obici S, Zhang BB, Karkanias G, Rossetti L. Hypothalamic insulin signaling is required for inhibition of glucose production. Nat Med. 2002; 8:1376–82.
Article5. Pocai A, Lam TK, Gutierrez-Juarez R, Obici S, Schwartz GJ, Bryan J, et al. Hypothalamic K(ATP) channels control hepatic glucose production. Nature. 2005; 434:1026–31.
Article6. Clegg DJ, Riedy CA, Smith KA, Benoit SC, Woods SC. Differential sensitivity to central leptin and insulin in male and female rats. Diabetes. 2003; 52:682–7.
Article7. Brown LM, Clegg DJ, Benoit SC, Woods SC. Intraventricular insulin and leptin reduce food intake and body weight in C57BL/6J mice. Physiol Behav. 2006; 89:687–91.
Article8. Buettner C, Pocai A, Muse ED, Etgen AM, Myers MG Jr, Rossetti L. Critical role of STAT3 in leptin’s metabolic actions. Cell Metab. 2006; 4:49–60.9. Morton GJ, Schwartz MW. Leptin and the central nervous system control of glucose metabolism. Physiol Rev. 2011; 91:389–411.
Article10. Mighiu PI, Yue JT, Filippi BM, Abraham MA, Chari M, Lam CK, et al. Hypothalamic glucagon signaling inhibits hepatic glucose production. Nat Med. 2013; 19:766–72.
Article11. Abraham MA, Yue JT, LaPierre MP, Rutter GA, Light PE, Filippi BM, et al. Hypothalamic glucagon signals through the KATP channels to regulate glucose production. Mol Metab. 2013; 3:202–8.
Article12. Sandoval DA, Bagnol D, Woods SC, D’Alessio DA, Seeley RJ. Arcuate glucagon-like peptide 1 receptors regulate glucose homeostasis but not food intake. Diabetes. 2008; 57:2046–54.
Article13. Taher J, Baker CL, Cuizon C, Masoudpour H, Zhang R, Farr S, et al. GLP-1 receptor agonism ameliorates hepatic VLDL overproduction and de novo lipogenesis in insulin resistance. Mol Metab. 2014; 3:823–33.14. Lam TK, Gutierrez-Juarez R, Pocai A, Rossetti L. Regulation of blood glucose by hypothalamic pyruvate metabolism. Science. 2005; 309:943–7.15. Chari M, Lam CK, Wang PY, Lam TK. Activation of central lactate metabolism lowers glucose production in uncontrolled diabetes and diet-induced insulin resistance. Diabetes. 2008; 57:836–40.
Article16. Parton LE, Ye CP, Coppari R, Enriori PJ, Choi B, Zhang CY, et al. Glucose sensing by POMC neurons regulates glucose homeostasis and is impaired in obesity. Nature. 2007; 449:228–32.
Article17. Pocai A, Lam TK, Obici S, Gutierrez-Juarez R, Muse ED, Arduini A, et al. Restoration of hypothalamic lipid sensing normalizes energy and glucose homeostasis in overfed rats. J Clin Invest. 2006; 116:1081–91.
Article18. Arrieta-Cruz I, Su Y, Knight CM, Lam TK, Gutierrez-Juarez R. Evidence for a role of proline and hypothalamic astrocytes in the regulation of glucose metabolism in rats. Diabetes. 2013; 62:1152–8.19. Su Y, Lam TK, He W, Pocai A, Bryan J, Aguilar-Bryan L, et al. Hypothalamic leucine metabolism regulates liver glucose production. Diabetes. 2012; 61:85–93.20. Blouet C, Schwartz GJ. Brainstem nutrient sensing in the nucleus of the solitary tract inhibits feeding. Cell Metab. 2012; 16:579–87.
Article21. Grill HJ, Hayes MR. Hindbrain neurons as an essential hub in the neuroanatomically distributed control of energy balance. Cell Metab. 2012; 16:296–309.
Article22. Cheng W, Gordian D, Ludwig MQ, Pers TH, Seeley RJ, Myers MG Jr. Hindbrain circuits in the control of eating behaviour and energy balance. Nat Metab. 2022; 4:826–35.
Article23. Wu Q, Lemus MB, Stark R, Bayliss JA, Reichenbach A, Lockie SH, et al. The temporal pattern of cfos activation in hypothalamic, cortical, and brainstem nuclei in response to fasting and refeeding in male mice. Endocrinology. 2014; 155:840–53.24. Duca FA, Waise TM, Peppler WT, Lam TK. The metabolic impact of small intestinal nutrient sensing. Nat Commun. 2021; 12:903.25. Waise TM, Dranse HJ, Lam TK. The metabolic role of vagal afferent innervation. Nat Rev Gastroenterol Hepatol. 2018; 15:625–36.
Article26. Yue JT, Abraham MA, Bauer PV, LaPierre MP, Wang P, Duca FA, et al. Inhibition of glycine transporter-1 in the dorsal vagal complex improves metabolic homeostasis in diabetes and obesity. Nat Commun. 2016; 7:13501.
Article27. Duca FA, Bauer PV, Hamr SC, Lam TK. Glucoregulatory relevance of small intestinal nutrient sensing in physiology, bariatric surgery, and pharmacology. Cell Metab. 2015; 22:367–80.
Article28. Yue JT, Abraham MA, LaPierre MP, Mighiu PI, Light PE, Filippi BM, et al. A fatty acid-dependent hypothalamic-DVC neurocircuitry that regulates hepatic secretion of triglyceride-rich lipoproteins. Nat Commun. 2015; 6:5970.29. Lam CK, Chari M, Rutter GA, Lam TK. Hypothalamic nutrient sensing activates a forebrain-hindbrain neuronal circuit to regulate glucose production in vivo. Diabetes. 2011; 60:107–13.30. Hackl MT, Furnsinn C, Schuh CM, Krssak M, Carli F, Guerra S, et al. Brain leptin reduces liver lipids by increasing hepatic triglyceride secretion and lowering lipogenesis. Nat Commun. 2019; 10:2717.
Article31. Benedict C, Kern W, Schultes B, Born J, Hallschmid M. Differential sensitivity of men and women to anorexigenic and memory-improving effects of intranasal insulin. J Clin Endocrinol Metab. 2008; 93:1339–44.
Article32. Frank-Podlech S, von Schnurbein J, Veit R, Heni M, Machann J, Heinze JM, et al. Leptin replacement reestablishes brain insulin action in the hypothalamus in congenital leptin deficiency. Diabetes Care. 2018; 41:907–10.
Article33. Dash S, Xiao C, Morgantini C, Koulajian K, Lewis GF. Intranasal insulin suppresses endogenous glucose production in humans compared with placebo in the presence of similar venous insulin concentrations. Diabetes. 2015; 64:766–74.34. Heni M, Wagner R, Kullmann S, Gancheva S, Roden M, Peter A, et al. Hypothalamic and striatal insulin action suppresses endogenous glucose production and may stimulate glucose uptake during hyperinsulinemia in lean but not in overweight men. Diabetes. 2017; 66:1797–806.
Article35. Filippi BM, Bassiri A, Abraham MA, Duca FA, Yue JT, Lam TK. Insulin signals through the dorsal vagal complex to regulate energy balance. Diabetes. 2014; 63:892–9.
Article36. Filippi BM, Yang CS, Tang C, Lam TK. Insulin activates Erk1/2 signaling in the dorsal vagal complex to inhibit glucose production. Cell Metab. 2012; 16:500–10.
Article37. LaPierre MP, Abraham MA, Yue JT, Filippi BM, Lam TK. Glucagon signalling in the dorsal vagal complex is sufficient and necessary for high-protein feeding to regulate glucose homeostasis in vivo. EMBO Rep. 2015; 16:1299–307.38. Lam CK, Chari M, Su BB, Cheung GW, Kokorovic A, Yang CS, et al. Activation of N-methyl-D-aspartate (NMDA) receptors in the dorsal vagal complex lowers glucose production. J Biol Chem. 2010; 285:21913–21.39. Kanoski SE, Zhao S, Guarnieri DJ, DiLeone RJ, Yan J, De Jonghe BC, et al. Endogenous leptin receptor signaling in the medial nucleus tractus solitarius affects meal size and potentiates intestinal satiation signals. Am J Physiol Endocrinol Metab. 2012; 303:E496–503.
Article40. Hayes MR, Skibicka KP, Grill HJ. Caudal brainstem processing is sufficient for behavioral, sympathetic, and parasympathetic responses driven by peripheral and hindbrain glucagon-like-peptide-1 receptor stimulation. Endocrinology. 2008; 149:4059–68.
Article41. Hayes MR, Leichner TM, Zhao S, Lee GS, Chowansky A, Zimmer D, et al. Intracellular signals mediating the food intake-suppressive effects of hindbrain glucagon-like peptide-1 receptor activation. Cell Metab. 2011; 13:320–30.
Article42. Alhadeff AL, Mergler BD, Zimmer DJ, Turner CA, Reiner DJ, Schmidt HD, et al. Endogenous glucagon-like peptide-1 receptor signaling in the nucleus tractus solitarius is required for food intake control. Neuropsychopharmacology. 2017; 42:1471–9.
Article43. Alhadeff AL, Grill HJ. Hindbrain nucleus tractus solitarius glucagon-like peptide-1 receptor signaling reduces appetitive and motivational aspects of feeding. Am J Physiol Regul Integr Comp Physiol. 2014; 307:R465–70.44. Bootcov MR, Bauskin AR, Valenzuela SM, Moore AG, Bansal M, He XY, et al. MIC-1, a novel macrophage inhibitory cytokine, is a divergent member of the TGF-beta superfamily. Proc Natl Acad Sci U S A. 1997; 94:11514–9.45. Zhang SY, Bruce K, Danaei Z, Li RJ, Barros DR, Kuah R, et al. Metformin triggers a kidney GDF15-dependent area postrema axis to regulate food intake and body weight. Cell Metab. 2023; 35:875–86.
Article46. Patel S, Alvarez-Guaita A, Melvin A, Rimmington D, Dattilo A, Miedzybrodzka EL, et al. GDF15 provides an endocrine signal of nutritional stress in mice and humans. Cell Metab. 2019; 29:707–18.
Article47. Campderros L, Moure R, Cairo M, Gavalda-Navarro A, Quesada-Lopez T, Cereijo R, et al. Brown adipocytes secrete GDF15 in response to thermogenic activation. Obesity (Silver Spring). 2019; 27:1606–16.
Article48. Mullican SE, Lin-Schmidt X, Chin CN, Chavez JA, Furman JL, Armstrong AA, et al. GFRAL is the receptor for GDF15 and the ligand promotes weight loss in mice and nonhuman primates. Nat Med. 2017; 23:1150–7.49. Emmerson PJ, Wang F, Du Y, Liu Q, Pickard RT, Gonciarz MD, et al. The metabolic effects of GDF15 are mediated by the orphan receptor GFRAL. Nat Med. 2017; 23:1215–9.
Article50. Hsu JY, Crawley S, Chen M, Ayupova DA, Lindhout DA, Higbee J, et al. Non-homeostatic body weight regulation through a brainstem-restricted receptor for GDF15. Nature. 2017; 550:255–9.
Article51. Yang L, Chang CC, Sun Z, Madsen D, Zhu H, Padkjaer SB, et al. GFRAL is the receptor for GDF15 and is required for the anti-obesity effects of the ligand. Nat Med. 2017; 23:1158–66.
Article52. Suriben R, Chen M, Higbee J, Oeffinger J, Ventura R, Li B, et al. Antibody-mediated inhibition of GDF15-GFRAL activity reverses cancer cachexia in mice. Nat Med. 2020; 26:1264–70.
Article53. Wang D, Townsend LK, DesOrmeaux GJ, Frangos SM, Batchuluun B, Dumont L, et al. GDF15 promotes weight loss by enhancing energy expenditure in muscle. Nature. 2023; 619:143–50.54. Sjoberg KA, Sigvardsen CM, Alvarado-Diaz A, Andersen NR, Larance M, Seeley RJ, et al. GDF15 increases insulin action in the liver and adipose tissue via a β-adrenergic receptor-mediated mechanism. Cell Metab. 2023; 35:1327–40.
Article55. Tsai VW, Manandhar R, Jorgensen SB, Lee-Ng KK, Zhang HP, Marquis CP, et al. The anorectic actions of the TGFβ cytokine MIC-1/GDF15 require an intact brainstem area postrema and nucleus of the solitary tract. PLoS One. 2014; 9:e100370.
Article56. Borner T, Arnold M, Ruud J, Breit SN, Langhans W, Lutz TA, et al. Anorexia-cachexia syndrome in hepatoma tumour-bearing rats requires the area postrema but not vagal afferents and is paralleled by increased MIC-1/GDF15. J Cachexia Sarcopenia Muscle. 2017; 8:417–27.
Article57. Tsai VW, Zhang HP, Manandhar R, Lee-Ng KK, Lebhar H, Marquis CP, et al. Treatment with the TGF-b superfamily cytokine MIC-1/GDF15 reduces the adiposity and corrects the metabolic dysfunction of mice with diet-induced obesity. Int J Obes (Lond). 2018; 42:561–71.
Article58. Li RJ, Chiu JF, Bruce K, Zhang SY, Barros DR, Yue JT, et al. A glucose-sensing mechanism with glucose transporter 1 and pyruvate kinase in the area postrema regulates hepatic glucose production in rats. J Biol Chem. 2023; 299:104633.59. Cheng W, Gonzalez I, Pan W, Tsang AH, Adams J, Ndoka E, et al. Calcitonin receptor neurons in the mouse nucleus tractus solitarius control energy balance via the non-aversive suppression of feeding. Cell Metab. 2020; 31:301–12.
Article60. Cavanaugh AR, Schwartz GJ, Blouet C. High-fat feeding impairs nutrient sensing and gut brain integration in the caudomedial nucleus of the solitary tract in mice. PLoS One. 2015; 10:e0118888.
Article61. Cheng W, Ndoka E, Hutch C, Roelofs K, MacKinnon A, Khoury B, et al. Leptin receptor-expressing nucleus tractus solitarius neurons suppress food intake independently of GLP1 in mice. JCI Insight. 2020; 5:e134359.
Article62. Qiu W, Hutch CR, Wang Y, Wloszek J, Rucker RA, Myers MG, et al. Multiple NTS neuron populations cumulatively suppress food intake. Elife. 2023; 12:e85640.
Article63. Zhang C, Kaye JA, Cai Z, Wang Y, Prescott SL, Liberles SD. Area postrema cell types that mediate nausea-associated behaviors. Neuron. 2021; 109:461–72.64. Zhang C, Vincelette LK, Reimann F, Liberles SD. A brainstem circuit for nausea suppression. Cell Rep. 2022; 39:110953.
Article65. Fulton S, Horn CC, Zhang C. Characterizing a new tool to manipulate area postrema GLP1R+ neurons across species. Physiol Behav. 2024; 276:114474.
Article66. Sabatini PV, Frikke-Schmidt H, Arthurs J, Gordian D, Patel A, Rupp AC, et al. GFRAL-expressing neurons suppress food intake via aversive pathways. Proc Natl Acad Sci U S A. 2021; 118:e2021357118.
Article67. Li RJ, Batchuluun B, Zhang SY, Abraham MA, Wang B, Lim YM, et al. Nutrient infusion in the dorsal vagal complex controls hepatic lipid and glucose metabolism in rats. iScience. 2021; 24:102366.
Article68. Borner T, Shaulson ED, Ghidewon MY, Barnett AB, Horn CC, Doyle RP, et al. GDF15 induces anorexia through nausea and emesis. Cell Metab. 2020; 31:351–62.69. Worth AA, Shoop R, Tye K, Feetham CH, D’Agostino G, Dodd GT, et al. The cytokine GDF15 signals through a population of brainstem cholecystokinin neurons to mediate anorectic signalling. Elife. 2020; 9:e55164.
Article70. Carter ME, Han S, Palmiter RD. Parabrachial calcitonin gene-related peptide neurons mediate conditioned taste aversion. J Neurosci. 2015; 35:4582–6.
Article71. Borner T, Workinger JL, Tinsley IC, Fortin SM, Stein LM, Chepurny OG, et al. Corrination of a GLP-1 receptor agonist for glycemic control without emesis. Cell Rep. 2020; 31:107768.
Article72. Ludwig MQ, Cheng W, Gordian D, Lee J, Paulsen SJ, Hansen SN, et al. A genetic map of the mouse dorsal vagal complex and its role in obesity. Nat Metab. 2021; 3:530–45.
Article73. Frikke-Schmidt H, Hultman K, Galaske JW, Jorgensen SB, Myers MG Jr, Seeley RJ. GDF15 acts synergistically with liraglutide but is not necessary for the weight loss induced by bariatric surgery in mice. Mol Metab. 2019; 21:13–21.74. Ghidewon M, Wald HS, McKnight AD, De Jonghe BC, Breen DM, Alhadeff AL, et al. Growth differentiation factor 15 (GDF15) and semaglutide inhibit food intake and body weight through largely distinct, additive mechanisms. Diabetes Obes Metab. 2022; 24:1010–20.75. Breit SN, Manandhar R, Zhang HP, Lee-Ng M, Brown DA, Tsai VW. GDF15 enhances body weight and adiposity reduction in obese mice by leveraging the leptin pathway. Cell Metab. 2023; 35:1341–55.
Article76. Zhang SY, Danaei Z, Bruce K, Chiu JF, Lam TK. Acute activation of GFRAL in the area postrema contributes to glucose regulation independent of weight. Diabetes. 2024; 73:426–33.
Article77. Diabetes Prevention Program Research Group. The 10-year cost-effectiveness of lifestyle intervention or metformin for diabetes prevention: an intent-to-treat analysis of the DPP/DPPOS. Diabetes Care. 2012; 35:723–30.78. Lee A, Morley JE. Metformin decreases food consumption and induces weight loss in subjects with obesity with type II non-insulin-dependent diabetes. Obes Res. 1998; 6:47–53.
Article79. Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002; 346:393–403.80. Lachin JM, Christophi CA, Edelstein SL, Ehrmann DA, Hamman RF, Kahn SE, et al. Factors associated with diabetes onset during metformin versus placebo therapy in the diabetes prevention program. Diabetes. 2007; 56:1153–9.
Article81. Diabetes Prevention Program Research Group, Knowler WC, Fowler SE, Hamman RF, Christophi CA, Hoffman HJ, et al. 10-Year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet. 2009; 374:1677–86.
Article82. Gerstein HC, Pare G, Hess S, Ford RJ, Sjaarda J, Raman K, et al. Growth differentiation factor 15 as a novel biomarker for metformin. Diabetes Care. 2017; 40:280–3.
Article83. Coll AP, Chen M, Taskar P, Rimmington D, Patel S, Tadross JA, et al. GDF15 mediates the effects of metformin on body weight and energy balance. Nature. 2020; 578:444–8.
Article84. Day EA, Ford RJ, Smith BK, Mohammadi-Shemirani P, Morrow MR, Gutgesell RM, et al. Metformin-induced increases in GDF15 are important for suppressing appetite and promoting weight loss. Nat Metab. 2019; 1:1202–8.85. Mirzadeh Z, Faber CL, Schwartz MW. Central nervous system control of glucose homeostasis: a therapeutic target for type 2 diabetes? Annu Rev Pharmacol Toxicol. 2022; 62:55–84.
Article86. Li H, Guglielmetti C, Sei YJ, Zilberter M, Le Page LM, Shields L, et al. Neurons require glucose uptake and glycolysis in vivo. Cell Rep. 2023; 42:112335.87. Lundgaard I, Li B, Xie L, Kang H, Sanggaard S, Haswell JD, et al. Direct neuronal glucose uptake heralds activity-dependent increases in cerebral metabolism. Nat Commun. 2015; 6:6807.
Article88. Maher F. Immunolocalization of GLUT1 and GLUT3 glucose transporters in primary cultured neurons and glia. J Neurosci Res. 1995; 42:459–69.
Article89. Pellerin L, Magistretti PJ. Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci U S A. 1994; 91:10625–9.90. Magistretti PJ, Pellerin L. Cellular bases of brain energy metabolism and their relevance to functional brain imaging: evidence for a prominent role of astrocytes. Cereb Cortex. 1996; 6:50–61.
Article91. Bergersen LH. Is lactate food for neurons?: comparison of monocarboxylate transporter subtypes in brain and muscle. Neuroscience. 2007; 145:11–9.
Article92. Brooks GA, Dubouchaud H, Brown M, Sicurello JP, Butz CE. Role of mitochondrial lactate dehydrogenase and lactate oxidation in the intracellular lactate shuttle. Proc Natl Acad Sci U S A. 1999; 96:1129–34.
Article93. Abraham MA, Rasti M, Bauer PV, Lam TK. Leptin enhances hypothalamic lactate dehydrogenase A (LDHA)-dependent glucose sensing to lower glucose production in high-fat-fed rats. J Biol Chem. 2018; 293:4159–66.
Article94. Chari M, Yang CS, Lam CK, Lee K, Mighiu P, Kokorovic A, et al. Glucose transporter-1 in the hypothalamic glial cells mediates glucose sensing to regulate glucose production in vivo. Diabetes. 2011; 60:1901–6.95. Bohland M, Matveyenko AV, Saberi M, Khan AM, Watts AG, Donovan CM. Activation of hindbrain neurons is mediated by portal-mesenteric vein glucosensors during slow-onset hypoglycemia. Diabetes. 2014; 63:2866–75.
Article96. Ritter S, Dinh TT. 2-Mercaptoacetate and 2-deoxy-D-glucose induce Fos-like immunoreactivity in rat brain. Brain Res. 1994; 641:111–20.
Article97. Ozawa Y, Arima H, Banno R, Ito Y, Goto M, Morishita Y, et al. Lesion of area postrema attenuated hyperphagic responses to glucoprivation, but not transcriptional activation of the neuropeptide Y gene in rats. Neuroreport. 2012; 23:673–5.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Centrifugal Influence on Gustatory Neurons in the Nucleus of the Solitary Tract
- Tyrosine Hydroxylase, Dopamine-beta-Hydroxylase and Phenylethanolamine-N-Methyltransferase Immunoreactive Neurons of the Medulla Oblongata in the Apodemus agrarius
- Immunohistochemical Studies on Calcitonin Gene-related Peptide Cell in Rat Brain
- Food Intake and Gut Hormones
- Emerging role of the brain in the homeostatic regulation of energy and glucose metabolism