Int J Stem Cells.
2024 Aug;17(3):224-235. 10.15283/ijsc23091.
Human Endometrial Regenerative Cells for Neurological Disorders: Hype or Hope?
- Affiliations
-
- 1Neuroscience Research Center, Mashhad University of Medical Sciences, Mashhad, Iran
- 2Department of Neuroscience, Faculty of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran
- 3Shefa Neuroscience Research Center, Khatam Alanbia Hospital, Tehran, Iran
- 4Student Research Committee, Faculty of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran
- 5Department of Neuroscience, Faculty of Advanced Technologies in Medicine, Iran University of Medical Sciences, Tehran, Iran
- KMID: 2558602
- DOI: http://doi.org/10.15283/ijsc23091
Abstract
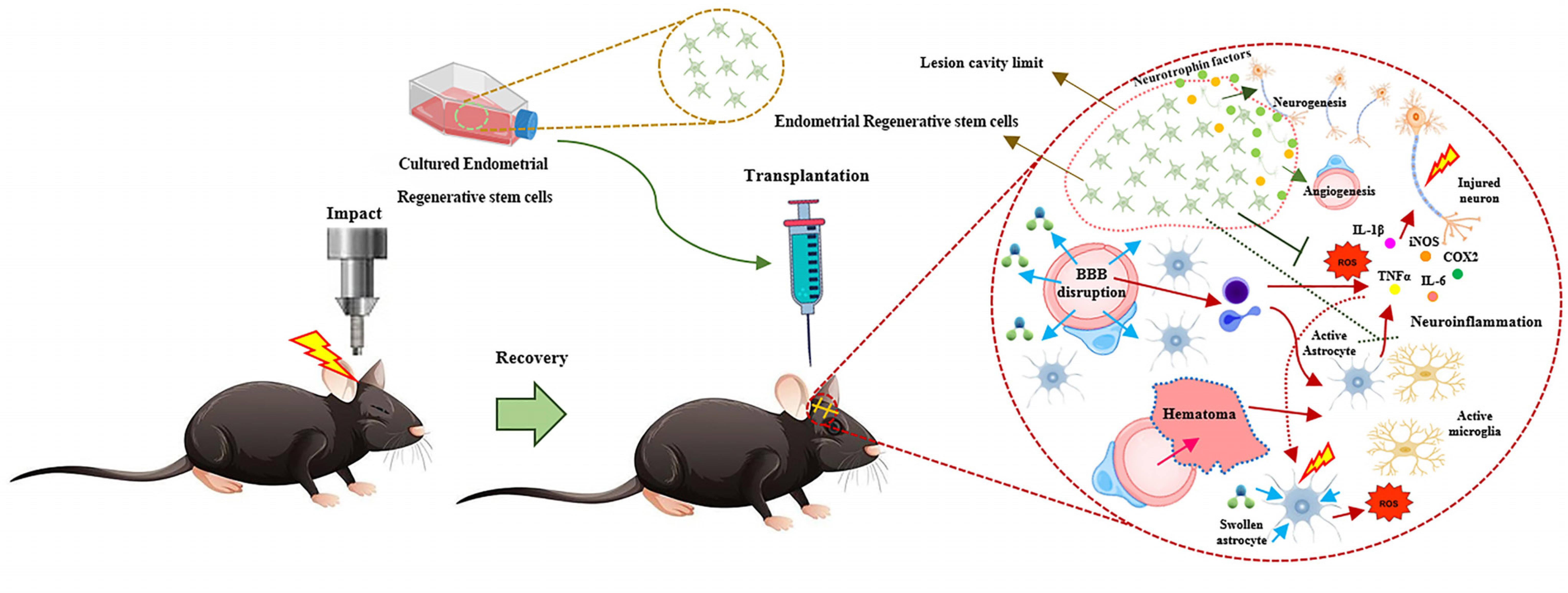
- Despite enormous efforts, no effective medication has been found to significantly halt or even slow the progression of neurological diseases, such as acquired (e.g., traumatic brain injury, spinal cord injury, etc.) and chronic (e.g., Parkinson’s disease, Alzheimer’s disease, etc.) central nervous system disorders. So, researchers are looking for alternative therapeutic modalities to manage the disease’s symptoms and stop it from worsening. Concerning disease-modifying capabilities, stem cell therapy has emerged as an expanding domain. Among different types of stem cells, human endometrial regenerative cells have excellent regenerative properties, making them suitable for regenerative medicine. They have the potential for self-renewal and differentiation into three types of stem cells: epithelial stem cells, endothelial side population stem cells, and mesenchymal stem cells (MSCs). ERCs can be isolated from endometrial biopsy and menstrual blood samples. However, there is no comprehensive evidence on the effects of ERCs on neurological disorders. Hence, we initially explore the traits of these specific stem cells in this analysis, followed by an emphasis on their therapeutic potential in treating neurological disorders.
Keyword
Figure
Reference
-
References
1. Feigin VL, Vos T, Nichols E, et al. 2020; The global burden of neurological disorders: translating evidence into policy. Lancet Neurol. 19:255–265. DOI: 10.1016/s1474-4422(19)30411-9. PMID: 31813850. PMCID: PMC9945815.2. De Gioia R, Biella F, Citterio G, et al. 2020; Neural stem cell transplantation for neurodegenerative diseases. Int J Mol Sci. 21:3103. DOI: 10.3390/ijms21093103. PMID: 32354178. PMCID: PMC7247151.3. Balestrino R, Schapira AHV. 2020; Parkinson disease. Eur J Neurol. 27:27–42. DOI: 10.1111/ene.14108. PMID: 31631455.4. Chiken S, Nambu A. 2016; Mechanism of deep brain stimulation: inhibition, excitation, or disruption? Neuroscientist. 22:313–322. DOI: 10.1177/1073858415581986. PMID: 25888630. PMCID: PMC4871171.5. Fox SH, Katzenschlager R, Lim SY, et al. 2011; The Movement Disorder Society evidence-based medicine review update: treatments for the motor symptoms of Parkinson’s disease. Mov Disord. 26 Suppl 3:S2–S41.6. Ahmadian-Moghadam H, Sadat-Shirazi MS, Zarrindast MR. 2020; Therapeutic potential of stem cells for treatment of neurodegenerative diseases. Biotechnol Lett. 42:1073–1101. DOI: 10.1007/s10529-020-02886-1. PMID: 32342435.7. Andrzejewska A, Dabrowska S, Lukomska B, Janowski M. 2021; Mesenchymal stem cells for neurological disorders. Adv Sci (Weinh). 8:2002944. DOI: 10.1002/advs.202002944. PMID: 33854883. PMCID: PMC8024997.8. Zakrzewski W, Dobrzyński M, Szymonowicz M, Rybak Z. 2019; Stem cells: past, present, and future. Stem Cell Res Ther. 10:68. DOI: 10.1186/s13287-019-1165-5. PMID: 30808416. PMCID: PMC6390367.9. Kolios G, Moodley Y. 2013; Introduction to stem cells and regenerative medicine. Respiration. 85:3–10. DOI: 10.1159/000345615. PMID: 23257690.10. Sobhani A, Khanlarkhani N, Baazm M, et al. 2017; Multipotent stem cell and current application. Acta Med Iran. 55:6–23. PMID: 28188938.11. Gurusamy N, Alsayari A, Rajasingh S, Rajasingh J. 2018; Adult stem cells for regenerative therapy. Prog Mol Biol Transl Sci. 160:1–22. DOI: 10.1016/bs.pmbts.2018.07.009. PMID: 30470288.12. Barkho BZ, Zhao X. 2011; Adult neural stem cells: response to stroke injury and potential for therapeutic applications. Curr Stem Cell Res Ther. 6:327–338. DOI: 10.2174/157488811797904362. PMID: 21466483. PMCID: PMC3199296.13. Nam H, Lee KH, Nam DH, Joo KM. 2015; Adult human neural stem cell therapeutics: current developmental status and prospect. World J Stem Cells. 7:126–136. DOI: 10.4252/wjsc.v7.i1.126. PMID: 25621112. PMCID: PMC4300923.14. Bozorgmehr M, Gurung S, Darzi S, et al. 2020; Endometrial and menstrual blood mesenchymal stem/stromal cells: biologi-cal properties and clinical application. Front Cell Dev Biol. 8:497. DOI: 10.3389/fcell.2020.00497. PMID: 32742977. PMCID: PMC7364758.15. Zuo W, Xie B, Li C, et al. 2018; The clinical applications of endometrial mesenchymal stem cells. Biopreserv Biobank. 16:158–164. DOI: 10.1089/bio.2017.0057. PMID: 29265881. PMCID: PMC5906727.16. Meng X, Ichim TE, Zhong J, et al. 2007; Endometrial regenera-tive cells: a novel stem cell population. J Transl Med. 5:57. DOI: 10.1186/1479-5876-5-57. PMID: 18005405. PMCID: PMC2212625.17. Jin W, Zhao Y, Hu Y, et al. 2020; Stromal cell-derived factor-1 enhances the therapeutic effects of human endometrial regenerative cells in a mouse sepsis model. Stem Cells Int. 2020:4820543. DOI: 10.1155/2020/4820543. PMID: 32256608. PMCID: PMC7103048.18. Kong Y, Shao Y, Ren C, Yang G. 2021; Endometrial stem/progenitor cells and their roles in immunity, clinical application, and endometriosis. Stem Cell Res Ther. 12:474. DOI: 10.1186/s13287-021-02526-z. PMID: 34425902. PMCID: PMC8383353.19. Zhong Z, Patel AN, Ichim TE, et al. 2009; Feasibility investi-gation of allogeneic endometrial regenerative cells. J Transl Med. 7:15. DOI: 10.1186/1479-5876-7-15. PMID: 19232091. PMCID: PMC2649897.20. Ichim TE, Alexandrescu DT, Solano F, et al. 2010; Mesenchymal stem cells as anti-inflammatories: implications for treatment of Duchenne muscular dystrophy. Cell Immunol. 260:75–82. DOI: 10.1016/j.cellimm.2009.10.006. PMID: 19917503.21. Wang Z, Wang D, Liu Y, et al. 2021; Mesenchymal stem cell in mice uterine and its therapeutic effect on osteoporosis. Rejuvenation Res. 24:139–150. DOI: 10.1089/rej.2019.2262. PMID: 32567490.22. Liu Y, Niu R, Li W, et al. 2019; Therapeutic potential of mens-trual blood-derived endometrial stem cells in cardiac diseases. Cell Mol Life Sci. 76:1681–1695. DOI: 10.1007/s00018-019-03019-2. PMID: 30721319. PMCID: PMC11105669.23. Chen L, Qu J, Xiang C. 2019; The multi-functional roles of menstrual blood-derived stem cells in regenerative medicine. Stem Cell Res Ther. 10:1. DOI: 10.1186/s13287-018-1105-9. PMID: 30606242. PMCID: PMC6318883.24. Peron JP, Jazedje T, Brandão WN, et al. 2012; Human endometrial-derived mesenchymal stem cells suppress inflam-mation in the central nervous system of EAE mice. Stem Cell Rev Rep. 8:940–952. DOI: 10.1007/s12015-011-9338-3. PMID: 22180029.25. Hong IS. 2022; Endometrial stem/progenitor cells: properties, origins, and functions. Genes Dis. 10:931–947. DOI: 10.1016/j.gendis.2022.08.009. PMID: 37396532. PMCID: PMC10308170.26. Maruyama T. 2014; Endometrial stem/progenitor cells. J Obstet Gynaecol Res. 40:2015–2022. DOI: 10.1111/jog.12501. PMID: 25160689.27. Li H, Yahaya BH, Ng WH, Yusoff NM, Lin J. 2019; Conditioned medium of human menstrual blood-derived endometrial stem cells protects against MPP+-induced cytotoxicity in vitro. Front Mol Neurosci. 12:80.28. Masuda H, Schwab KE, Filby CE, et al. 2021; Endometrial stem/progenitor cells in menstrual blood and peritoneal fluid of women with and without endometriosis. Reprod Biomed Online. 43:3–13. DOI: 10.1016/j.rbmo.2021.04.008. PMID: 34011465.29. Mobarakeh ZT, Ai J, Yazdani F, et al. 2012; Human endometrial stem cells as a new source for programming to neural cells. Cell Biol Int Rep (2010). 19:e00015. DOI: 10.1042/cbr20110009. PMID: 23124318. PMCID: PMC3475442.30. Liu Y, Niu R, Yang F, et al. 2018; Biological characteristics of human menstrual blood-derived endometrial stem cells. J Cell Mol Med. 22:1627–1639. DOI: 10.1111/jcmm.13437. PMID: 29278305. PMCID: PMC5824373.31. Cheng Y, Li L, Wang D, et al. 2017; Characteristics of human endometrium-derived mesenchymal stem cells and their tropism to endometriosis. Stem Cells Int. 2017:4794827. DOI: 10.1155/2017/4794827. PMID: 28761446. PMCID: PMC5518492.32. Fayazi M, Salehnia M, Ziaei S. 2015; Differentiation of human CD146-positive endometrial stem cells to adipogenic-, osteogenic-, neural progenitor-, and glial-like cells. In Vitro Cell Dev Biol Anim. 51:408–414. DOI: 10.1007/s11626-014-9842-2. PMID: 25515247.33. Kojour MA, Ebrahimi-Barough S, Kouchesfehani HM, Jalali H, Ebrahim MH. 2017; Oleic acid promotes the expression of neural markers in differentiated human endometrial stem cells. J Chem Neuroanat. 79:51–57. DOI: 10.1016/j.jchemneu.2016.11.004. PMID: 27865908.34. Noureddini M, Verdi J, Mortazavi-Tabatabaei SA, et al. 2012; Human endometrial stem cell neurogenesis in response to NGF and bFGF. Cell Biol Int. 36:961–966. DOI: 10.1042/cbi20110610. PMID: 22804708.35. Wolff EF, Gao XB, Yao KV, et al. 2011; Endometrial stem cell transplantation restores dopamine production in a Parki-nson’s disease model. J Cell Mol Med. 15:747–755.36. Zhao Y, Chen X, Wu Y, Wang Y, Li Y, Xiang C. 2018; Trans-plantation of human menstrual blood-derived mesenchymal stem cells alleviates Alzheimer’s disease-like pathology in APP/PS1 transgenic mice. Front Mol Neurosci. 11:140.37. Borlongan CV, Kaneko Y, Maki M, et al. 2010; Menstrual blood cells display stem cell-like phenotypic markers and exert neuroprotection following transplantation in experimental stroke. Stem Cells Dev. 19:439–452. DOI: 10.1089/scd.2009.0340. PMID: 19860544. PMCID: PMC3158424.38. Wu Q, Wang Q, Li Z, et al. 2018; Human menstrual blood-derived stem cells promote functional recovery in a rat spinal cord hemisection model. Cell Death Dis. 9:882. DOI: 10.1038/s41419-018-0847-8. PMID: 30158539. PMCID: PMC6115341.39. Shi Y, Liu Y, Zhang B, Li X, Lin J, Yang C. 2023; Human menstrual blood-derived endometrial stem cells promote functional recovery by improving the inflammatory microenvi-ronment in a mouse spinal cord injury model. Cell Trans-plant. 32:9636897231154579. DOI: 10.1177/09636897231154579. PMID: 36786359. PMCID: PMC9932767.40. Wolff EF, Mutlu L, Massasa EE, Elsworth JD, Eugene Redmond D Jr, Taylor HS. 2015; Endometrial stem cell transplantation in MPTP- exposed primates: an alternative cell source for treatment of Parkinson’s disease. J Cell Mol Med. 19:249–256.41. Yang X, Devianti M, Yang YH, et al. 2019; Endometrial mesenchymal stem/stromal cell modulation of T cell prolifera-tion. Reproduction. 157:43–52. DOI: 10.1530/REP-18-0266. PMID: 30392200.42. Manganeli Polonio C, Longo de Freitas C, Garcia de Oliveira M, et al. 2021; Murine endometrial-derived mesenchymal stem cells suppress experimental autoimmune encephalomyelitis depending on indoleamine-2,3-dioxygenase expression. Clin Sci (Lond). 135:1065–1082. DOI: 10.1042/cs20201544. PMID: 33960391.43. León-Moreno LC, Castañeda-Arellano R, Aguilar-García IG, et al. 2020; Kinematic changes in a mouse model of penetrating hippocampal injury and their recovery after intranasal administration of endometrial mesenchymal stem cell-derived extracellular vesicles. Front Cell Neurosci. 14:579162. DOI: 10.3389/fncel.2020.579162. PMID: 33192324. PMCID: PMC7533596.44. Hasanzadeh E, Ebrahimi-Barough S, Mahmoodi N, et al. 2021; Defining the role of 17β-estradiol in human endometrial stem cells differentiation into neuron-like cells. Cell Biol Int. 45:140–153. DOI: 10.1002/cbin.11478. PMID: 33049079.45. Brown RH, Al-Chalabi A. 2017; Amyotrophic lateral sclerosis. N Engl J Med. 377:162–172. DOI: 10.1056/nejmra1603471. PMID: 28700839.46. Shirian S, Ebrahimi-Barough S, Saberi H, et al. 2016; Compari-son of capability of human bone marrow mesenchymal stem cells and endometrial stem cells to differentiate into motor neurons on electrospun poly (ε-caprolactone) scaffold. Mol Neurobiol. 53:5278–5287. DOI: 10.1007/s12035-015-9442-5. PMID: 26420037.47. Ebrahimi-Barough S, Hoveizi E, Yazdankhah M, et al. 2017; Inhibitor of PI3K/Akt signaling pathway small molecule promotes motor neuron differentiation of human endome-trial stem cells cultured on electrospun biocomposite polycaprolactone/collagen scaffolds. Mol Neurobiol. 54:2547–2554. DOI: 10.1007/s12035-016-9828-z. PMID: 26993294.48. Ebrahimi-Barough S, Norouzi Javidan A, Saberi H, et al. 2015; Evaluation of motor neuron-like cell differentiation of hEnSCs on biodegradable PLGA nanofiber scaffolds. Mol Neurobiol. 52:1704–1713. DOI: 10.1007/s12035-014-8931-2. PMID: 25377792.49. Mahmoodi N, Ai J, Ebrahimi-Barough S, et al. 2020; Microtubule stabilizer epothilone B as a motor neuron differentiation agent for human endometrial stem cells. Cell Biol Int. 44:1168–1183. DOI: 10.1002/cbin.11315. PMID: 32022385.50. Mohamadi F, Ebrahimi-Barough S, Nourani MR, et al. 2018; Enhanced sciatic nerve regeneration by human endometrial stem cells in an electrospun poly (ε-caprolactone)/collagen/NBG nerve conduit in rat. Artif Cells Nanomed Biotechnol. 46:1731–1743. DOI: 10.1080/21691401.2017.1391823. PMID: 29117721.51. Jalali Monfared M, Nasirinezhad F, Ebrahimi-Barough S, et al. 2019; Transplantation of miR-219 overexpressed human endometrial stem cells encapsulated in fibrin hydrogel in spinal cord injury. J Cell Physiol. 234:18887–18896. DOI: 10.1002/jcp.28527. PMID: 30982976.52. Babaloo H, Ebrahimi-Barough S, Derakhshan MA, et al. 2019; PCL/gelatin nanofibrous scaffolds with human endometrial stem cells/Schwann cells facilitate axon regeneration in spinal cord injury. J Cell Physiol. 234:11060–11069. DOI: 10.1002/jcp.27936. PMID: 30584656.53. Terraf P, Kouhsari SM, Ai J, Babaloo H. 2017; Tissue-engineered regeneration of hemisected spinal cord using human endometrial stem cells, poly ε-caprolactone scaffolds, and crocin as a neuroprotective agent. Mol Neurobiol. 54:5657–5667. DOI: 10.1007/s12035-016-0089-7. PMID: 27624387.