J Korean Med Sci.
2024 Aug;39(32):e235. 10.3346/jkms.2024.39.e235.
First Nationwide Mpox Vaccination Program in the Republic of Korea: Implications for an Enhanced Public Health Response
- Affiliations
-
- 1Division of Immunization, Korea Disease Control and Prevention Agency, Cheongju, Korea
- 2KDI School of Public Policy and Management, Sejong, Korea
- 3Graduate School of Public Health, Chungnam National University, Daejeon, Korea
- 4Division of Emerging Infectious Disease Response, Bureau of Infectious Disease Emergency Preparedness and Response, Korea Disease Control and Prevention Agency, Cheongju, Korea
- 5Division of Vaccine Supply, Korea Disease Control and Prevention Agency, Cheongju, Korea
- 6Bureau of Healthcare Safety and Immunization, Korea Disease Control and Prevention Agency, Cheongju, Korea
- KMID: 2558532
- DOI: http://doi.org/10.3346/jkms.2024.39.e235
Abstract
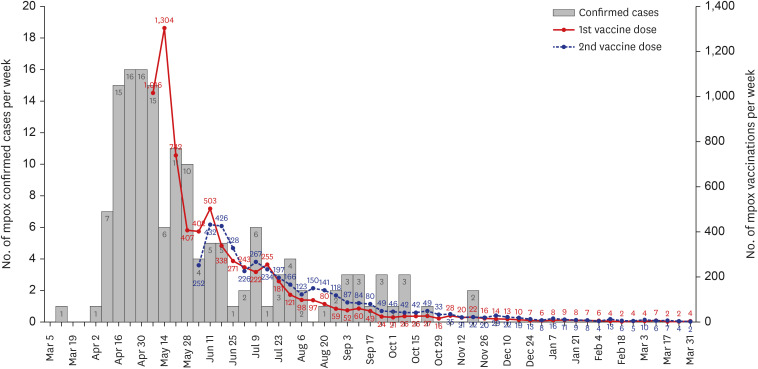
- On May 1, 2024, the Republic of Korea lifted the infectious disease crisis alert for mpox, almost two years after the first case was reported. The Korea Disease Control and Prevention Agency (KDCA) has led the response, which included diagnosis, epidemiological investigations, treatment, and vaccination. This article particularly reviews the vaccination strategy implemented and proposes suggestions for enhancing future response efforts. Initially, the KDCA recommended pre-exposure prophylaxis for high-risk groups, later expanding to include broader demographics as domestic cases rose. By April 2024, a total of 6,863 individuals had received their first vaccine dose, with 3,875 completing the second dose of third-generation vaccines. Strategies to improve future responses include addressing stigma, securing nationally representative safety data, and conducting vaccine cost-benefit analyses. These measures will help ensure a robust and effective response to future outbreaks.
Keyword
Figure
Reference
-
1. Jang YR, Lee M, Shin H, Kim JW, Choi MM, Kim YM, et al. The first case of monkeypox in the Republic of Korea. J Korean Med Sci. 2022; 37(27):e224. PMID: 35818706.2. Lee SY, Park YJ, Lee HM. The establishment of the monkeypox response system and the result of the initial response. Public Health Weekly Report. 2022; 15(34):2423–2431.3. Kwon SL, Ban S, Shin J, Bae H, Park H, Kwon GY. Monkeypox vaccination in the Republic of Korea: identifying the high-risk target group. J Korean Med Sci. 2022; 37(29):e239. PMID: 35880509.4. Lim SY, Jung YM, Kim Y, Kim G, Jeon J, Chin B, et al. Adverse reactions after intradermal vaccination with JYNNEOS for mpox in Korea. J Korean Med Sci. 2024; 39(8):e100. PMID: 38442725.5. Kim T, Park E, Kim J, Shim MG, Lee S, Kim E. Effective responses to mpox (monkeypox): epidemiologic investigation and foreign policy. Public Health Wkly Rep. 2023; 16(22):669–683.6. Petersen E, Zumla A, Hui DS, Blumberg L, Valdoleiros SR, Amao L, et al. Vaccination for monkeypox prevention in persons with high-risk sexual behaviours to control on-going outbreak of monkeypox virus clade 3. Int J Infect Dis. 2022; 122:569–571. PMID: 35788415.7. Huang MF, Chang YP, Lin CW, Yen CF. Factors related to mpox-vaccine uptake among men who have sex with men in Taiwan: roles of information sources and emotional problems. Vaccines (Basel). 2024; 12(3):332. PMID: 38543965.8. Owens LE, Currie DW, Kramarow EA, Siddique S, Swanson M, Carter RJ, et al. JYNNEOS vaccination coverage among persons at risk for mpox - United States, May 22, 2022–January 31, 2023. MMWR Morb Mortal Wkly Rep. 2023; 72(13):342–347. PMID: 36995962.9. Deng L, Lopez LK, Glover C, Cashman P, Reynolds R, Macartney K, et al. Short-term adverse events following immunization with modified vaccinia Ankara-Bavarian Nordic (MVA-BN) vaccine for mpox. JAMA. 2023; 329(23):2091–2094. PMID: 37145654.10. Zheng M, Du M, Yang G, Yao Y, Qian X, Zhi Y, et al. Mpox vaccination hesitancy and its associated factors among men who have sex with men in China: a national observational study. Vaccines (Basel). 2023; 11(9):1432. PMID: 37766109.11. Valfort MA. Society at a Glance: a Spotlight on LGBT People. Paris, France: OECD;2019.12. Lim SY, Jo HJ, Lee SY, Ahn M, Kim Y, Jeon J, et al. Clinical features of mpox patients in Korea: a multicenter retrospective study. J Korean Med Sci. 2024; 39(4):e19. PMID: 38288533.13. Kuehn R, Fox T, Guyatt G, Lutje V, Gould S. Infection prevention and control measures to reduce the transmission of mpox: a systematic review. PLOS Glob Public Health. 2024; 4(1):e0002731. PMID: 38236835.14. Taub DD, Ershler WB, Janowski M, Artz A, Key ML, McKelvey J, et al. Immunity from smallpox vaccine persists for decades: a longitudinal study. Am J Med. 2008; 121(12):1058–1064. PMID: 19028201.15. Lee J, Kwon SL, Park J, Bae H, Lee H, Kwon GY. JYNNEOS vaccine safety monitoring in the Republic of Korea, 2022: a cross-sectional study. Osong Public Health Res Perspect. 2023; 14(5):433–438. PMID: 37920899.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Adverse Reactions After Intradermal Vaccination With JYNNEOS for Mpox in Korea
- JYNNEOS vaccine safety monitoring in the Republic of Korea, 2022: a cross-sectional study
- Twenty Years of Progress and the Way Forward: Immunization Registry Information System in Korea
- Challenges in capacity building of national immunization programs and emergency or pandemic vaccination responses in the Global Health Security Agenda member countries
- A Case of Human Mpox with Isolated Perianal Ulcers Development in Convalescent Phase